Network Topology of ASD Risk Genes: From Genetic Architecture to Therapeutic Discovery
This article synthesizes current research on the network topology of Autism Spectrum Disorder (ASD) risk genes, addressing a core challenge in the field: bridging the gap between hundreds of identified...
Network Topology of ASD Risk Genes: From Genetic Architecture to Therapeutic Discovery
Abstract
This article synthesizes current research on the network topology of Autism Spectrum Disorder (ASD) risk genes, addressing a core challenge in the field: bridging the gap between hundreds of identified genetic associations and a coherent understanding of the disorder's underlying biology. We explore the foundational genetic architecture of ASD, highlighting the shift from single-gene to polygenic and network-based perspectives. The content details advanced computational methodologies, including network propagation and co-expression analysis, that are used to pinpoint biologically relevant gene modules and core regulatory hubs from complex genomic data. We further address the critical challenge of heterogeneity by examining strategies for deconvolving ASD into more genetically homogeneous subtypes and optimizing network models for enhanced predictive power. Finally, we evaluate the translational potential of these approaches, showcasing how network-based findings are being validated and applied to identify novel biomarkers and reposition existing drugs for ASD treatment. This resource is designed for researchers, scientists, and drug development professionals seeking to leverage systems biology for breakthroughs in ASD etiology and therapy.
The Genetic Landscape and Network Architecture of Autism Spectrum Disorder
The genetic architecture of autism spectrum disorder (ASD) represents a complex continuum spanning rare, high-penetrance mutations and common, small-effect polygenic variation. This technical review synthesizes contemporary evidence from large-scale genomic studies to delineate the oligogenic model of ASD liability, wherein additive and interactive effects across variant classes determine individual risk and phenotypic outcomes. We provide quantitative comparisons of effect sizes and population attributable risks, detailed experimental methodologies for variant detection and burden analysis, and visualizations of genetic networks. For drug development professionals, this work highlights pathway convergence points and the critical importance of patient stratification based on genetic profiles for targeted therapeutic intervention.
Autism spectrum disorder (ASD) exemplifies the complexity of neurodevelopmental conditions, with its genetic underpinnings comprising multiple classes of risk variants operating through diverse biological mechanisms. Historically, research approaches have bifurcated into the study of rare variants with large effects and common variants with individually small effects, but contemporary models recognize their synergistic interplay in determining liability [1] [2]. This technical guide comprehensively synthesizes current understanding of ASD's oligogenic architecture, framing evidence within the context of risk genes network topology research.
The emerging paradigm rejects simplistic dichotomies in favor of a multifactorial model where de novo mutations (DNMs), inherited rare variants, and polygenic risk backgrounds collectively shape neurodevelopmental trajectories [1]. Recent studies employing person-centered phenotypic decomposition have revealed that distinct clinical presentations correlate with specific genetic programs, enabling more precise mapping of genotype-phenotype relationships [3]. Furthermore, evidence indicates that age at diagnosis reflects divergent developmental trajectories with distinct genetic profiles, underscoring the temporal dimension of genetic risk manifestation [4].
For researchers and therapeutic developers, understanding this architectural complexity is paramount for target identification, clinical trial design, and patient stratification strategies. This review integrates quantitative genetic findings with methodological guidance and network-based analytical frameworks to advance these applications.
The Spectrum of Genetic Contributions to ASD Liability
ASD liability arises from the integrated contribution of multiple variant classes with differing effect sizes and population frequencies. The table below quantifies the key parameters for major genetic risk categories.
Table 1: Quantitative Profile of Major Genetic Risk Variants in ASD
| Variant Class | Effect Size (OR/RRR) | Population Attributable Fraction | Key Characteristics | Associated Clinical Features |
|---|---|---|---|---|
| Rare De Novo Mutations (protein-truncating) | 2.5-20+ [1] | ~5-10% [1] | Arise spontaneously in germline; negative selection | Lower adaptive functioning, greater behavioral symptoms relative to family background [5] |
| Inherited Rare Variants (private, LGD) | 1.5-3.0 [1] | ~10-15% (estimated) | Transmitted across generations; often oligogenic | More likely in multiplex families; older mutational origin (2-3 generations) [1] |
| Common Variants (polygenic) | 1.05-1.15 per allele [2] | 40-50% [2] | Aggregate in polygenic scores; additive effects | Later diagnosis; increased socioemotional difficulties in adolescence [4] |
| Rare CNVs | 2.0-10.0 [1] | ~5% | Recurrent deletions/duplications; variable expressivity | Intellectual disability, developmental delays [6] |
The additive model of liability accumulation is supported by empirical evidence showing that ASD subjects carrying rare potentially damaging variants (PDVs) still carry a significant burden of common risk variants—intermediate between non-carrier ASD subjects and control subjects [2]. This indicates that common polygenic risk contributes to liability even in the presence of major rare variants.
Table 2: Developmental Trajectories and Genetic Correlations by Diagnosis Age
| Developmental Profile | Typical Age at Diagnosis | Polygenic Correlation with ADHD/Mental Health Conditions | Early Social/Communication Abilities | Socioemotional Trajectory |
|---|---|---|---|---|
| Early Childhood Emergent | Earlier diagnosis (by age 5-7) | Moderate genetic correlations [4] | Lower abilities in early childhood [4] | Stable or modestly attenuating difficulties [4] |
| Late Childhood Emergent | Later diagnosis (mid-childhood to adolescence) | Moderate to high positive genetic correlations [4] | Fewer difficulties in early childhood [4] | Increasing difficulties in late childhood/adolescence [4] |
Methodological Approaches for Variant Detection and Burden Analysis
Whole Exome/Genome Sequencing for De Novo Mutation Detection
Experimental Protocol: DNM identification requires trio-based design (proband + both parents) with high-quality sequencing.
- DNA Preparation: Extract DNA from whole blood (Korean, SSC cohorts) or saliva (SPARK cohort) using standardized protocols [5].
- Sequencing Platforms: Utilize Illumina platforms (NovaSeq 6000 for SPARK WES; HiSeq X10 for SSC WGS; HiSeq X for Korean WGS) [5].
- Variant Calling Pipeline:
- Align reads to GRCh38 using BWA-mem
- Process with GATK following best practices (v4.1.8.1 for Korean WES; v3.5 for SPARK/SSC)
- Apply variant quality score recalibration (VQSR)
- Perform joint genotyping with iterative gVCF genotyper (Korean WGS) or GLnexus (v1.4.1 for SPARK) [5]
- DNV Identification: Use Hail 0.2 de_novo() function with allele frequency <0.01% in gnomAD v3.1 non-neuro population [5].
- Quality Filtering:
- Heterozygous SNPs: QUAL ≥7.5, GQmean ≥36, DPmean ≥34, allele balance 0.275-0.725
- Heterozygous indels: QUAL ≥10.51, gDP ≥3, AB 0.214-0.786 [5]
- Variant Annotation: Apply Hail's vep() function with Ensembl VEP v109.3; classify as protein-truncating (PTV), missense (MIS), or synonymous [5].
Polygenic Risk Scoring and G-BLUP Analysis
Experimental Protocol: Common variant burden assessment for case-control and family-based designs.
- Genotyping and Quality Control:
- Genotype on Illumina platforms (Infinium OmniExpressExome-8 for PAGES; Human1M for SSC)
- Impute at Michigan Imputation Server using HRC reference panel [2]
- Apply post-imputation QC: exclude variants with R² < 0.3, MAF < 0.01
- LD Pruning: Use PLINK 2.0 with --clump-r² 0.81; --clump-kb 50 to obtain ~910K SNPs [2].
- PRS Calculation:
- Obtain GWAS summary statistics from ASD meta-analyses
- Clump SNPs to remove LD (r² < 0.1 within 250kb windows)
- Calculate scores as weighted sum of risk alleles: ( PRS = \sum{i}βi × Gi ) where (βi) is effect size and (G_i) is allele count [2]
- G-BLUP Implementation:
- Use genomic relationship matrix (GRM) from ~550K LD-pruned SNPs
- Apply mixed linear models to predict ASD status: ( y = Xβ + Zu + ε ) where u ~ N(0, Gσ²g) [2]
- Tune parameters via cross-validation within reference population
Within-Family Phenotype Deviation Analysis
Experimental Protocol: Control for familial background in phenotype-genotype mapping.
- Phenotype Standardization: Collect standardized measures of ASD core symptoms (Social Communication Questionnaire) and adaptive functioning (Vineland) [5].
- WFSD Calculation: Compute within-family standardized deviation: ( WFSD = \frac{(P{proband} - μ{unaffected})}{σ{unaffected}} ) where (μ{unaffected}) and (σ_{unaffected}) are mean and standard deviation of unaffected family members' scores [5].
- Outlier Analysis: Identify genes with high intrafamilial variability using per-gene WFSD distributions; apply statistical cutoffs (e.g., Z > 2.5) [5].
- Mutation Site Mapping: Corregate phenotypic heterogeneity with functional domains (e.g., voltage-sensing vs. pore-forming domains in SCN2A) [5].
Research Reagent Solutions
Table 3: Essential Research Reagents and Resources for ASD Genetic Studies
| Reagent/Resource | Specifications | Application in ASD Genetics | Example Use Cases |
|---|---|---|---|
| Illumina Sequencing Platforms | NovaSeq 6000, HiSeq X10, HiSeq X | Whole genome/exome sequencing of trios | DNM discovery in SSC, SPARK, Korean cohorts [5] |
| Genotyping Arrays | Infinium OmniExpressExome-8, Human1M, HumanOmni-2.5 | Common variant profiling in large cohorts | Polygenic scoring in PAGES, SSC [2] |
| GRCh38 Reference Genome | Genome Reference Consortium human build 38 | Unified reference for alignment and variant calling | All major consortium studies (SPARK, SSC, MSSNG) [5] |
| HRC Reference Panel | Haplotype Reference Consortium, ~65,000 haplotypes | Genotype imputation for common variants | Imputation at Michigan Imputation Server [2] |
| gnomAD Database | v3.1 non-neuro subset | Population allele frequency filtering | DNM identification (AF < 0.01%) [5] |
| STRING Database | v11, minimum score 0.9 threshold | Protein-protein interaction network construction | Interactome generation for PTHS [7] |
| Cytoscape with MCODE | v3.9.1, MCODE plug-in | Network module identification | Molecular complex detection in co-expression networks [7] |
| WGCNA R Library | v1.72-1, minimum module size 30 | Co-expression network analysis | Module identification in neural differentiation data [7] |
Network Topology of ASD Risk Genes
The convergence of ASD risk genes into functional networks represents a fundamental insight from systems biology approaches. Protein-protein interaction and co-expression analyses reveal that seemingly heterogeneous genetic risk factors coalesce into coordinated biological programs.
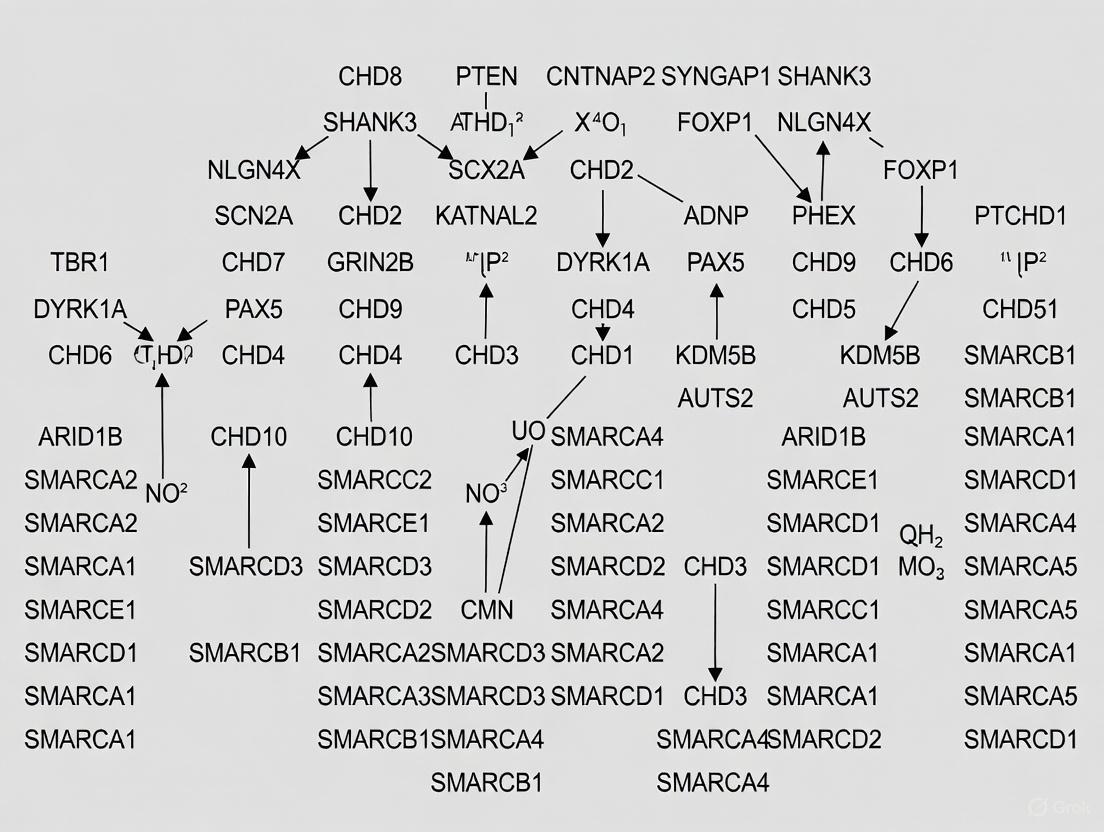
Figure 1: Integrated Network of ASD Genetic Risk Convergence. Multiple genetic risk classes converge on molecular networks that drive cellular phenotypes and clinical manifestations.
Analysis of Pitt-Hopkins syndrome (PTHS), a monogenic form of ASD caused by TCF4 mutations, reveals striking network topology through co-expression analysis in neural progenitor cells (NPCs) and neurons. The PTHS interactome demonstrates temporal specificity, with NPC networks enriched for upregulated genes (325 nodes, 504 edges; hypergeometric test p = 5.05e-34) while neuronal networks show predominant downregulation (673 nodes, 1897 edges; p = 7.58e-49) [7]. This temporal dynamic illustrates how ASD risk genes operate in developmentally regulated programs.
Hub gene analysis further identifies central nodes within co-expression modules, including:
- Histone gene family members - associated with neuronal differentiation pathways
- Synaptic vesicle trafficking proteins - regulating neurotransmission and connectivity
- Cell signaling mediators - integrating developmental cues [7]
These hub genes represent potential therapeutic targets as their central network positions exert disproportionate influence on overall system behavior.
Implications for Therapeutic Development
The oligogenic architecture of ASD necessitates precision medicine approaches for therapeutic development. Several strategic implications emerge:
Patient Stratification Biomarkers
Genetic profiling enables identification of biologically coherent subgroups with distinct pathway disruptions. Phenotypic decomposition analyses reveal four clinically distinct classes with characteristic genetic profiles: Social/behavioral, Mixed ASD with DD, Moderate challenges, and Broadly affected [3]. Each class demonstrates unique co-occurring condition patterns and developmental trajectories, suggesting differential treatment responses.
Target Prioritization Framework
The network topology of ASD risk genes provides a rational framework for target prioritization. Hub genes within co-expression modules represent particularly influential nodes whose modulation may produce cascading effects through entire functional networks [7]. For example, histone modification genes identified in PTHS analyses offer epigenetic intervention points.
Developmental Timing Considerations
The efficacy of targeted interventions likely depends on developmental windows. Genes associated with earlier ASD diagnosis show distinct expression patterns from those with later diagnosis [4], suggesting that treatments targeting these pathways may have age-dependent efficacy. Network analyses revealing temporal shifts in gene expression between NPC and neuronal stages further support this concept [7].
The genetic architecture of ASD embodies a complex, multilayered model where rare and common variants collectively determine liability through additive effects on convergent biological pathways. This oligogenic model supersedes earlier dichotomous frameworks and provides a more nuanced foundation for understanding disease mechanisms and advancing therapeutic development. The integration of person-centered phenotypic analysis with network biology approaches offers particularly promising directions for identifying coherent subgroups and their associated genetic programs. For drug development professionals, these advances enable more precise patient stratification, target identification, and clinical trial design aligned with the underlying biological heterogeneity of ASD.
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition with a strong genetic basis. Research over the past decade has revealed that its pathogenesis involves hundreds of risk genes converging onto a limited set of biological pathways. Network-based analyses of these genes have proven particularly valuable for elucidating ASD pathophysiology, moving beyond single-gene approaches to system-level understanding. This whitepaper examines three core pathways—synaptic function, chromatin remodeling, and transcriptional regulation—that emerge consistently from network topology studies of ASD risk genes. By integrating findings from genomic, transcriptomic, and proteomic analyses, we provide a comprehensive technical overview of these pathways, their interconnections, and their implications for therapeutic development.
Synaptic Function and Signaling Pathways
Synaptic dysfunction represents one of the most consistently implicated mechanisms in ASD pathogenesis. Network analyses of ASD risk genes reveal significant enrichment for genes encoding proteins essential for synaptic formation, function, and plasticity.
Key Synaptic Pathways
Glutamatergic Signaling: Genes encoding glutamate receptors (GRIN2B) and postsynaptic density proteins (SHANK3) are frequently disrupted in ASD. These proteins mediate excitatory synaptic transmission and are crucial for learning and memory. Alterations in the balance between excitatory and inhibitory signaling have been proposed as a fundamental mechanism underlying ASD-related behaviors. Impaired NMDA receptor-dependent long-term potentiation (LTP) and enhanced long-term depression (LTD) have been observed in multiple ASD models, suggesting disrupted synaptic plasticity mechanisms [8] [9].
GABAergic Signaling: Genes involved in GABA synthesis (GAD), transport, and receptor function (GABRB3) show altered expression in ASD. These disruptions affect inhibitory neurotransmission, potentially contributing to the excitatory/inhibitory imbalance observed in ASD neural circuits. Postmortem studies have revealed significantly reduced levels of glutamic acid decarboxylase and GABAA and GABAB receptor alterations in brains of individuals with autism [9].
Neurexin-Neuroligin Pathway: These cell adhesion molecules organize presynaptic and postsynaptic domains and mediate trans-synaptic signaling. Mutations in genes encoding these proteins (NLGN3, NLGN4, NRXN1) disrupt synaptic connectivity and are among the most robustly associated with ASD. These proteins form a trans-synaptic bridge that organizes both sides of the synapse and regulates the balance of excitatory and inhibitory neurotransmission [10] [9].
Synaptic Multi-Omics Analysis
Recent advances in synaptosome analysis have enabled detailed molecular characterization of synaptic components in ASD. Integrated multi-omics approaches analyzing miRNAs, mRNAs, and proteins in synaptosomes from post-mortem brain tissues have revealed significant alterations in ASD.
Table 1: Synaptosome Multi-Omics Analysis in ASD
| Analysis Type | Sample Characteristics | Key Findings | Technical Approach |
|---|---|---|---|
| miRNA-Seq | 27 AD, 14 controls | Upregulation of miRNA-501-3p, miRNA-502-3p, miRNA-877-5p with AD progression | Total RNA extraction with TriZol; LC Sciences HiSeq |
| mRNA-Seq | 27 AD, 14 controls | Hundreds of differentially expressed mRNAs affecting microglia, astrocytes, electron transport chain | Poly(A) RNA sequencing; Illumina NovaSeq 6000 |
| Proteomic Analysis | 27 AD, 14 controls | Alterations in Calsyntenin-1, GluR2, GluR4, Neurexin-2A | Mass spectrometry of synaptosomal proteins |
Integrated analysis revealed complex relationships between deregulated synaptic miRNAs and their target mRNAs and proteins, demonstrating the impact of deregulated miRNAs on synaptic function in ASD. DIABLO analysis showed intricate relationships among mRNAs, miRNAs, and proteins that could be key in understanding synaptic pathophysiology [8].
Chromatin Remodeling Mechanisms
Chromatin remodeling refers to the dynamic adjustment of chromatin structure through the action of enzyme complexes that change nucleosome positioning, composition, and accessibility. Network analyses have identified significant enrichment of chromatin remodeling genes among high-confidence ASD risk genes.
Chromatin Remodeling Complexes in ASD
ATP-dependent chromatin remodeling complexes utilize ATP hydrolysis to alter nucleosome positions and modify histone-DNA interactions. These complexes are categorized into four major families based on their catalytic subunits and functional mechanisms.
Table 2: Chromatin Remodeling Complexes Implicated in ASD
| Complex Family | Key Subunits | Mechanism of Action | ASD Association |
|---|---|---|---|
| SWI/SNF | ARID1B, SMARCA2, SMARCA4 | Nucleosome sliding, eviction; chromatin decompaction | De novo LoF mutations in ARID1B, SMARCA2 |
| CHD/NuRD | CHD8, CHD7 | Nucleosome repositioning, histone deacetylation | CHD8 among strongest ASD risk genes |
| ISWI | SMARCA1, BAZ1B | Nucleosome spacing, chromatin compaction | Implicated in ASD via network analyses |
| INO80 | INO80, YY1 | Histone variant exchange (H2A.Z) | YY1 mutations associated with ASD |
These complexes regulate the accessibility of DNA to transcription factors and RNA polymerase, thereby controlling gene expression programs critical for neurodevelopment. Their disruption in ASD alters the transcriptional landscape of developing neurons, affecting processes such as neuronal differentiation, migration, and connectivity [10] [11] [12].
Mechanisms of Chromatin Remodeling
Nucleosome Sliding: Remodeling complexes use ATP hydrolysis to break histone-DNA contacts and reposition nucleosomes along DNA, exposing or concealing regulatory sequences.
Nucleosome Eviction: Complete removal of nucleosomes from specific DNA regions creates persistently accessible chromatin domains.
Histone Variant Exchange: Replacement of canonical histones with specialized variants (e.g., H2A.Z) alters nucleosome stability and properties.
Histone Modification: Covalent modifications (acetylation, methylation) of histone tails influence chromatin compaction and protein recruitment. Notably, genes encoding histone-modifying enzymes (KATNAL2, SUV420H1, ASH1L, MLL3) are recurrently mutated in ASD, particularly those involved in histone lysine methylation and demethylation [10] [12].
Transcriptional Regulation Networks
Transcriptional regulation represents a central hub in ASD gene networks, with numerous risk genes encoding transcription factors, coactivators, and components of the transcriptional machinery.
Transcriptional Complexes and Pioneer Factors
The process of transcriptional activation involves sequential recruitment of coregulatory complexes to gene regulatory elements. Chromatin modifiers play essential roles in this process by altering chromatin structure to facilitate transcription.
Pioneer Factors: Specialized transcription factors capable of binding compacted chromatin and initiating chromatin decompaction. Examples include FoxA and GATA factors, which can bind their target sequences in nucleosomal DNA and recruit additional factors. These factors exhibit cooperative relationships with nuclear receptors; for instance, FoxA1 and ERα bind DNA cooperatively, with each capable of pioneering chromatin access depending on cellular context [13].
Core Transcriptional Machinery: The basal transcription apparatus, including RNA polymerase II and associated general transcription factors, is recruited to promoters following chromatin remodeling.
Mediator Complex: A multi-subunit complex that bridges transcription factors with the RNA polymerase II apparatus, integrating signals from various regulatory elements.
Transcriptional Dysregulation in ASD
Network analyses have identified several key transcriptional regulators as high-confidence ASD risk genes, including TBR1, BCL11A, and MYT1L. These transcription factors often function at the top of regulatory hierarchies controlling neurodevelopmental gene expression programs. The convergence of ASD risk genes on specific transcriptional networks is evidenced by protein-protein interaction modules strongly enriched for autism candidate genes, with members exhibiting unusual evolutionary constraint against mutations [10].
ASD-associated mutations in transcriptional regulators disrupt gene expression programs essential for proper brain development, including neuronal specification, migration, and connectivity. These disruptions alter the transcriptomic landscape of the developing brain, ultimately affecting neural circuit formation and function [9].
Network Analysis Methods and Experimental Protocols
Network-based approaches provide powerful tools for identifying convergent pathways from diverse ASD genetic risk factors. These methods leverage protein-protein interactions, gene co-expression patterns, and functional annotations to detect modules enriched for ASD risk genes.
Gene Correlation Network Analysis
Sample Preparation:
- Utilize gene expression profiles from relevant tissues (e.g., peripheral blood lymphocytes or brain regions)
- Dataset: GSE25507 from NCBI with 82 autistic patients and 64 healthy controls
- Each sample contains 23,520 genes
- Data preprocessing with MAS5 and RMA algorithms
Network Construction:
- Establish Spearman correlation networks for both case and control groups
- Analyze structural parameters (average degree) under different thresholds
- Identify genes with significant differences in average degree between groups (MD-Gs)
Functional Analysis:
- Annotate top MD-Gs with significant structural differences
- Perform enrichment analysis for biological processes and pathways
- Validate findings against known ASD pathways and mechanisms [14]
Integrated Multi-Omics Analysis
Synaptosome Preparation:
- Extract synaptosomes from post-mortem brain samples (Brodmann's Area 10 of frontal cortices)
- Use Syn-PER Reagent with Dounce glass homogenization on ice
- Centrifuge at 1400g for 10min at 4°C, then collect supernatant
- Recentrifuge supernatant at 15,000g for 20min at 4°C to obtain synaptosome pellet
- Characterize isolated synaptosomes by transmission electron microscopy
Multi-Omics Profiling:
- Extract total RNA including miRNAs using TriZol reagent
- Perform miRNA and mRNA sequencing commercially (LC Sciences)
- Conduct proteomic analysis of synaptosomal proteins via mass spectrometry
- Integrate datasets using DIABLO analysis to identify multimodal molecular signatures [8]
Statistical Genetics Approach
Variant Calling and Annotation:
- Use exome sequencing data from 3,871 ASD cases and 9,937 controls
- Call SNVs and indels using GATK (v2.6) in a single large batch
- Identify de novo mutations with enhanced calling methods
- Annotate variants by type (de novo, case, control, transmitted, non-transmitted) and severity (LoF, damaging missense)
Gene-Based Association Testing:
- Apply TADA (Transmission and De novo Association) statistical model
- Integrate de novo, transmitted, and case-control variation
- Calculate gene-level Bayes Factors and False Discovery Rate q-values
- Validate findings using orthogonal approaches (e.g., female-male frequency differences) [10]
Pathway Visualization
Synaptic Dysfunction in ASD
Chromatin Remodeling in ASD
Research Reagent Solutions
Table 3: Essential Research Reagents for ASD Pathway Investigation
| Reagent/Category | Specific Examples | Application in ASD Research |
|---|---|---|
| Sequencing Kits | Illumina TruSeq stranded mRNA kit | Library preparation for transcriptomic studies of ASD models |
| Antibodies | Anti-SHANK3, Anti-CHD8, Anti-PSD-95 | Protein expression analysis in postmortem brain tissues and cellular models |
| Cell Lines | iPSC-derived neurons from ASD patients | Modeling patient-specific mutations in synaptic and chromatin pathways |
| Animal Models | Shank3 KO, Chd8 heterozygous, Fmr1 KO mice | In vivo functional validation of ASD risk genes and pathways |
| Chromatin Assays | ATAC-seq, ChIP-seq kits | Profiling chromatin accessibility and histone modifications in ASD |
| Synaptosome Isolation | Syn-PER Reagent | Isolation of synaptic fractions for proteomic and transcriptomic analysis |
| Bioinformatic Tools | TADA, STRING, Cytoscape | Network analysis of ASD risk genes and pathway convergence |
Network analysis of ASD risk genes has systematically identified synaptic function, chromatin remodeling, and transcriptional regulation as three principal pathways disrupted in autism spectrum disorder. These pathways do not operate in isolation but exhibit significant crosstalk, forming an interconnected network that orchestrates neurodevelopment. The convergence of genetic risk factors onto these core pathways provides a framework for understanding ASD pathophysiology and developing targeted interventions. Future research should aim to further elucidate the temporal dynamics of these pathway disruptions across development and their specific roles in different neural circuits. The continued refinement of network-based approaches, combined with multi-omics profiling of well-characterized cohorts, will likely yield additional insights into ASD biology and identify novel therapeutic targets.
The autism spectrum disorder (ASD) interactome represents a comprehensive map of protein-protein interactions (PPIs) that form the molecular basis of neurodevelopmental processes. In the context of ASD risk gene research, network topology provides a crucial framework for understanding how seemingly disparate genetic risk factors converge on shared biological pathways. ASD is characterized by profound genetic heterogeneity, with hundreds of risk genes identified through sequencing studies, yet these genes consistently converge on specific functional networks and biological processes [15] [16]. The interactome concept moves beyond single-gene analysis to reveal how mutations in different genes can disrupt interconnected protein networks, ultimately leading to common pathological outcomes in ASD.
Recent advances in network medicine have demonstrated that proteins encoded by ASD risk genes do not operate in isolation but rather form functional modules within larger biological networks. These modules represent groups of proteins that work together to execute specific cellular functions, and their disruption provides critical insights into ASD pathophysiology. The application of interactome mapping in ASD research has revealed that the topological properties of risk genes within protein networks—including their connectivity, centrality, and relationship to network hubs—can illuminate fundamental disease mechanisms and identify novel therapeutic targets [16] [17].
Methodological Approaches for Interactome Mapping
Experimental Techniques for PPI Mapping
Proximity-Dependent Labeling in Neuronal Models
BioID2 (Proximity-Dependent Biotin Identification) has emerged as a powerful technique for mapping PPIs in cell-type-specific contexts. In this method, a promiscuous biotin ligase is fused to a protein of interest (bait), which then biotinylates proximate proteins in living cells. The biotinylated proteins can subsequently be purified and identified via mass spectrometry. This approach has been successfully applied to map interactions for 41 ASD risk genes in primary mouse neurons, revealing convergent pathways including mitochondrial processes, Wnt signaling, and MAPK signaling [17]. The key advantage of BioID2 is its ability to capture weak and transient interactions in live cells under physiological conditions.
Immunoprecipitation-Mass Spectrometry (IP-MS) in Human Neurons
IP-MS in induced excitatory neurons represents another robust approach for mapping the ASD interactome. This technique involves expressing ASD risk genes in human stem-cell-derived neurogenin-2 induced excitatory neurons (iNs), followed by immunoprecipitation of the index proteins and identification of interactors via liquid chromatography and tandem mass spectrometry (LC-MS/MS) [15] [18]. A landmark study applying this methodology to 13 high-confidence ASD risk genes identified more than 1,000 interactions, approximately 90% of which were novel, underscoring the importance of cell-type-specific protein interaction mapping [15]. The workflow typically includes validation steps through Western blotting and assessment of interaction reproducibility, with successful applications demonstrating greater than 80% replication rates.
Computational and Network-Based Approaches
Network Diffusion and Module Detection
Network diffusion-based methods analyze the propagation of genetic signals through molecular interaction networks to identify disease-relevant modules. These approaches leverage the "guilt-by-association" principle, positing that genes causing similar phenotypes tend to interact physically or functionally [16]. The network smoothing index (NSI) quantifies the network relevance of each gene in relation to a set of input ASD risk genes, considering the whole network while mitigating the excessive influence of highly connected hubs [16]. This method has proven particularly valuable for integrating multiple, non-overlapping ASD risk gene lists from different studies and identifying significantly connected gene modules associated with ASD.
Machine Learning and Gene Prioritization
Machine learning approaches integrate diverse data types—including spatiotemporal gene expression patterns from human brain development, gene-level constraint metrics, and network features—to predict novel ASD risk genes [19]. These methods typically employ supervised learning algorithms trained on known ASD risk genes from resources like SFARI (Simons Foundation Autism Research Initiative) and use features such as brain region-specific co-expression patterns, protein-protein interaction network topologies, and evolutionary constraint metrics. Validation studies demonstrate that genes identified through these prediction models show enrichment in independent sets of ASD risk genes and tend to be dysregulated in postmortem ASD brains [19].
Key Findings from ASD Interactome Studies
Convergent Biological Pathways in ASD
Interactome mapping studies have consistently identified several key biological pathways that represent points of convergence for multiple ASD risk genes.
Table 1: Key Pathways Dysregulated in ASD Identified Through Interactome Mapping
| Pathway | ASD Risk Genes Involved | Biological Function | Experimental Evidence |
|---|---|---|---|
| Synaptic Transmission | Multiple genes encoding synaptic scaffolding, vesicle trafficking, and neurotransmitter receptor proteins | Regulation of neuronal communication, synaptic plasticity, and neurotransmitter release | IP-MS in human iNs shows enrichment for synaptic proteins [15] [18] |
| Wnt Signaling | Proteins involved in canonical and non-canonical Wnt pathways | Regulation of neuronal differentiation, axon guidance, and synapse formation | BioID2 in primary neurons identifies Wnt components [17] |
| mTOR Signaling | PTEN, TSC1/2, and associated regulators | Control of cell growth, protein synthesis, and metabolism | Network analysis reveals mTOR pathway enrichment [15] |
| Chromatin Remodeling | CHD8, ARID1B, and other chromatin regulators | Epigenetic regulation of gene expression during neurodevelopment | Co-expression modules show chromatin remodeling enrichment [19] |
| Mitochondrial Function | Genes encoding mitochondrial proteins and metabolic regulators | Cellular energy production, oxidative stress response, and metabolism | BioID2 shows association between ASD risk genes and mitochondrial activity [17] |
| GABAergic Signaling | G protein subunits, GABA receptors, and associated proteins | Primary inhibitory neurotransmission in CNS | In silico analysis implicates G proteins in GABAergic pathways [20] [21] |
Network Topology of ASD Risk Genes
Table 2: Network Topology Properties of ASD Risk Genes
| Topological Property | Description | Research Findings | Citation |
|---|---|---|---|
| Degree Centrality | Number of direct interactions for a protein in the network | ASD risk proteins show variable connectivity, with some acting as hubs (e.g., DYRK1A with 604 interactors) and others having limited connections (e.g., PTEN with 3 interactors) | [15] |
| Betweenness Centrality | Measure of a protein's role as a connector between different network modules | Proteins with high betweenness may represent critical points of network vulnerability in ASD | [16] |
| Module Membership | Assignment of proteins to functionally related clusters | ASD risk genes cluster into distinct modules reflecting biological pathways (synaptic function, chromatin remodeling, etc.) | [17] [19] |
| Evolutionary Constraint | Tolerance to functional genetic variation | ASD risk genes show significant intolerance to protein-disrupting mutations (high pLI scores) | [19] |
Signaling Pathways in ASD Revealed by Interactome Mapping
G Protein-Coupled Receptor Signaling Pathways
Recent interactome studies have revealed dysregulation of G protein subunits in ASD pathophysiology. Experimental evidence shows altered serum levels of specific G protein subunits in individuals with ASD compared to controls, with significantly decreased GNAO1 and significantly increased GNAI1 levels observed [20] [21]. In silico analysis of the interaction networks involving these G protein subunits implicates them in GABAergic and dopamine signaling pathways, both critically involved in the neurobiological basis of ASD [21]. These findings suggest that dysregulation of G protein signaling pathways may represent a convergent mechanism in ASD.
IGF2BP Complex as a Convergent Network Node
Interactome studies in human induced neurons have identified the insulin-like growth factor 2 mRNA-binding proteins (IGF2BP1-3) as a highly interconnected complex within the ASD protein network. These proteins, which together form an m6A-reader complex, each interact with at least five ASD index proteins, suggesting they may function as major mediators in convergent biological pathways for ASD risk [15] [18]. This complex potentially regulates a transcriptional circuit of ASD-associated genes, representing a point of functional integration for multiple genetic risk factors.
Clinical and Translational Applications
ASD Subtyping Based on Network Pathology
Recent large-scale studies have leveraged interactome data to identify biologically distinct subtypes of ASD. By analyzing data from over 5,000 children in the SPARK cohort and employing computational models that consider combinations of clinical traits and genetic profiles, researchers have defined four clinically and biologically distinct subtypes of autism [22]:
- Social and Behavioral Challenges Group (37%): Characterized by core autism traits without developmental delays, but with frequent co-occurring conditions like ADHD, anxiety, and depression.
- Mixed ASD with Developmental Delay (19%): Features developmental milestones delays but typically without anxiety, depression, or disruptive behaviors.
- Moderate Challenges (34%): Presents with milder core autism-related behaviors and typical developmental milestone achievement.
- Broadly Affected (10%): Displays wide-ranging challenges including developmental delays, social-communication difficulties, and co-occurring psychiatric conditions.
Each subtype demonstrates distinct genetic profiles, with the Broadly Affected group showing the highest proportion of damaging de novo mutations, while the Mixed ASD with Developmental Delay group is more likely to carry rare inherited genetic variants [22]. This subtyping approach enables more precise mapping of genetic risk factors to specific clinical presentations.
Drug Target Identification
Interactome mapping facilitates network-based drug target discovery by identifying proteins that occupy critical positions within dysregulated pathways. For example, the identification of the IGF2BP complex as a convergent node suggests that modulating its function could potentially impact multiple ASD risk pathways simultaneously [15] [18]. Similarly, the delineation of specific G protein signaling abnormalities points to potential targets for pharmacological intervention [20] [21]. The ability to cluster risk genes based on PPI networks has also been shown to identify gene groups corresponding to clinical behavior score severity, enabling more targeted therapeutic development [17].
Research Reagent Solutions for Interactome Studies
Table 3: Essential Research Reagents for ASD Interactome Mapping
| Reagent/Tool | Application | Key Features | Example Use Cases |
|---|---|---|---|
| BioID2 System | Proximity-dependent labeling in live cells | Promiscuous biotin ligase for labeling proximate proteins, works in neuronal cells | Mapping protein interactions for 41 ASD risk genes in primary neurons [17] |
| IP-MS Platform | Protein complex isolation and identification | Antibody-based purification followed by LC-MS/MS identification | Identifying >1,000 interactions for 13 ASD genes in human iNs [15] [18] |
| STRING Database | Protein-protein interaction prediction | Integrates known and predicted PPIs from multiple sources | Building interactomes for network diffusion analysis [16] [20] |
| BrainSpan Atlas | Spatiotemporal gene expression reference | Transcriptomic data across human brain development and regions | Machine learning prediction of ASD risk genes [19] |
| SFARI Gene Database | Curated ASD risk gene resource | Categorizes genes by evidence strength for ASD association | Training and validation sets for prediction models [19] |
| Human iN Differentiation Protocol | Generation of excitatory neurons from stem cells | Neurogenin-2 induction for consistent excitatory neuron production | Cell-type-specific PPI mapping for ASD risk genes [15] [18] |
Future Directions
The evolving field of ASD interactome research is increasingly moving toward integration of multi-omics data and cell-type-specific network mapping. Future efforts will likely focus on expanding PPI networks to include more ASD risk genes across different neuronal cell types (e.g., inhibitory neurons, glial cells) and developmental time points. The combination of interactome data with other data modalities—including transcriptomics, epigenomics, and clinical information—holds promise for developing comprehensive network models that can predict disease trajectories and treatment responses. Additionally, the application of single-cell proteomics and spatial transcriptomics to ASD research will likely provide unprecedented resolution in understanding the cell-type-specific organization of protein networks disrupted in ASD.
As these technologies advance, interactome mapping will increasingly inform precision medicine approaches for ASD, enabling clinicians to match individuals with specific network pathologies to targeted interventions. The continued refinement of ASD subtypes based on underlying biological mechanisms, coupled with network-based drug discovery, represents a promising pathway toward more effective, personalized treatments for autism spectrum disorder.
The genetic architecture of Autism Spectrum Disorder (ASD) is exceptionally complex, characterized by high heritability estimates of 64-91% alongside significant heterogeneity [23]. Unraveling this complexity requires sample sizes orders of magnitude larger than those available to individual research institutions. This necessity has driven the formation of international consortia and large-scale genomic resources that aggregate data across multiple research sites. The Simons Foundation Powering Autism Research for Knowledge (SPARK) consortium, for example, represents a massive effort to collect and analyze genetic data from over 50,000 individuals with ASD, with current genomic data available for more than 115,000 participants, including over 44,000 with autism who have undergone whole exome sequencing [24]. Similarly, the Autism Sequencing Consortium (ASC) brings together international scientists who share ASD samples and genetic data, facilitating joint analysis of large-scale data from many groups [25]. These collaborative frameworks have enabled researchers to overcome previous limitations in statistical power, leading to the identification of hundreds of genetic loci significantly associated with ASD risk and providing insights into the biological pathways disrupted in the condition [26] [27].
The value of these resources extends beyond mere data aggregation. They provide standardized phenotypic characterization, implement rigorous quality control procedures, and develop innovative analytical frameworks that enable the research community to explore genotype-phenotype relationships at unprecedented resolution. The MSSNG resource, for instance, has recently expanded to include whole-genome sequencing (WGS) data from 5,100 individuals with ASD and 6,212 non-ASD family members, facilitating comprehensive examination of the roles of many types of genetic variation in ASD, including common single nucleotide polymorphisms (SNPs), rare and de novo single nucleotide variants (SNVs), short insertions/deletions (indels), mitochondrial DNA (mtDNA) variants, and structural variants (SVs) [23]. This expanded scope allows researchers to move beyond a narrow focus on protein-coding regions to investigate the full genomic landscape of ASD.
Table 1: Major Genomic Resources for ASD Research
| Resource | Sample Size | Data Types | Key Features |
|---|---|---|---|
| SPARK | >115,000 participants (44,000+ with ASD WES) [24] | WES, WGS, SNP array | Diverse genetic ancestry; family-based design; integration with phenotypic data |
| MSSNG | 11,312 individuals (5,100 with ASD) [23] | WGS (GRCh38) | Comprehensive variant calls (SNVs, CNVs, SVs, TREs); cloud-based data access via Google Cloud Platform |
| ASC | >50,000 exomes planned [25] | WES, WGS | International collaboration; coordinated analysis across sites; focus on rare variants |
| iPSYCH-PGC | >18,000 individuals with ASD [28] | GWAS | Population-based cohort; integration with national registries; common variant focus |
Key Findings from Genomic Studies
Common Variant Associations through GWAS
Genome-wide association studies (GWAS) have identified specific common genetic variants contributing to ASD risk, though their individual effect sizes are typically small. A recent GWAS on 6,222 case-pseudocontrol pairs from the SPARK dataset identified one novel genome-wide significant (GWS) locus and four significant loci through meta-analysis with previous studies [28]. The previously discovered three GWS ASD susceptibility loci from the iPSYCH-PGC study together explain only 0.13% of the liability for autism risk, whereas all common variants are estimated to explain 11.8% of liability [28], indicating that numerous additional common risk variants remain to be discovered.
Functional follow-up of GWAS findings has provided crucial insights into the biological mechanisms through which common variants influence ASD risk. For the novel locus identified in the SPARK GWAS, researchers employed a massively parallel reporter assay (MPRA) and identified a putative causal variant (rs7001340) with strong impacts on gene regulation [28]. Expression quantitative trait loci (eQTL) data demonstrated an association between the risk allele and decreased expression of DDHD2 (DDHD domain containing 2) in both adult and prenatal brains, establishing DDHD2 as a novel gene associated with ASD risk [28]. This work exemplifies the progression from genetic association to biological mechanism that is becoming increasingly possible with large sample sizes.
More recent findings have revealed that the polygenic architecture of autism can be decomposed into genetically correlated factors that align with clinical heterogeneity. A 2025 study demonstrated that common genetic variants account for approximately 11% of the variance in age at autism diagnosis and can be broken down into two modestly genetically correlated (rg = 0.38) autism polygenic factors [4]. One factor associates with earlier autism diagnosis and lower social and communication abilities in early childhood, while the second links to later autism diagnosis and increased socioemotional and behavioral difficulties in adolescence, with differential genetic correlations with other neurodevelopmental conditions [4].
Rare Variant Contributions through WES/WGS
Whole exome and whole genome sequencing studies have dramatically expanded the catalog of rare variants contributing to ASD risk. The latest release of the MSSNG resource has enabled the identification of ASD-associated rare variants in 14.1% of individuals with ASD from MSSNG and 14.5% from the Simons Simplex Collection (SSC) [23]. In terms of genomic architecture, 52% were nuclear sequence-level variants, 46% were nuclear structural variants, and 2% were mitochondrial variants [23]. This comprehensive assessment demonstrates that structural variants contribute nearly as much as sequence-level variants to ASD genetic risk, highlighting the importance of analyzing all variant types.
By incorporating de novo variants from 12,375 additional trios from MSSNG and SPARK into the ASC's TADA+ analysis, researchers have identified 134 ASD-associated genes with false discovery rate (FDR) <0.1, including 67 new genes not previously associated with ASD [23]. Notably, 27 of these new genes are not currently in the Simons Foundation Autism Research Initiative (SFARI) Gene database, providing novel molecules for study [23]. The evidence for most new genes constituted a mix of de novo protein-truncating variants (PTVs), de novo damaging missense (DMis) variants, and excess PTVs in cases compared with controls, though some genes showed evidence exclusively of PTVs (e.g., MED13, TANC2, DMWD) or de novo DMis variants (e.g., ATP2B2, DMPK, PAPOLG) [23]. This provides insight into potential molecular mechanisms, with haploinsufficiency suggested as a common mechanism for PTV-biased genes and gain-of-function or dominant-negative mechanisms for DMis-biased genes.
Table 2: Variant Types Identified in ASD Genomic Studies
| Variant Category | Specific Types | Contribution to ASD Risk | Detection Method |
|---|---|---|---|
| Common Variants | Single nucleotide polymorphisms (SNPs) | ~11.8% of liability [28] | GWAS |
| Rare Coding Variants | Protein-truncating variants (PTVs), Damaging missense (DMis) | Identified in 14.1-14.5% of ASD cases [23] | WES, WGS |
| Structural Variants | Copy number variants (CNVs), inversions, large insertions, uniparental isodisomies | 46% of rare variant findings [23] | WGS, microarray |
| Other Variants | Tandem repeat expansions (TREs), mitochondrial variants | ~2% of rare variant findings [23] | Specialized WGS analysis |
Gene Networks and Biological Pathways
Integration of genomic findings with network analysis approaches has revealed that ASD-risk genes converge on specific biological processes and pathways. Protein-protein interaction (PPI) network analysis of differentially expressed genes in ASD has identified key hub genes including SHANK3, NLRP3, SERAC1, TUBB2A, MGAT4C, TFAP2A, EVC, GABRE, TRAK1, and GPR161 [26]. These genes display high connectivity within molecular networks and have demonstrated strong discriminatory power in differentiating ASD from controls, particularly MGAT4C (AUC = 0.730) [26].
Network-based analyses leverage the principle of "guilt by association," where the function of an unannotated protein may be similar to that of its neighbors in a network if many of those neighbors are annotated with the same function [29]. Dense interconnections in protein interaction networks are characteristic of protein complexes or pathways, enabling identification of both known complexes and novel components of known systems [29]. In the context of ASD, such approaches have revealed abnormalities in key biological pathways involved in synaptic function, chromatin remodeling, and transcriptional regulation [26].
Recent work has also linked genetic findings to specific phenotypic presentations through person-centered approaches. Using generative mixture modeling on broad phenotypic data from 5,392 individuals in the SPARK cohort, researchers identified four clinically relevant classes of ASD that demonstrate distinct patterns of core, associated, and co-occurring traits [3]. These phenotypic classes show correspondence to genetic and molecular programs of common, de novo and inherited variation, with class-specific differences in the developmental timing of affected genes aligning with clinical outcome differences [3]. This represents a significant advance beyond trait-centric approaches that marginalize co-occurring phenotypes.
Methodological Approaches and Experimental Protocols
Genome-Wide Association Study (GWAS) Protocol
The standard protocol for GWAS in ASD research involves several key steps, as exemplified by the SPARK consortium approach [28]. First, genotype data undergoes rigorous quality control, including removal of individuals with call rates below a predetermined threshold and exclusion of monozygotic twins. Phasing is performed using algorithms such as EAGLE v2.4.1, followed by imputation using reference panels like the Trans-Omics for Precision Medicine (TOPMed) Freeze 5b, which consists of 125,568 haplotypes from multiple ancestries [28]. For family-based designs like SPARK, pseudocontrols are generated by selecting the alleles not inherited from parents to cases using PLINK 1.9 [28].
Association testing typically employs generalized linear models implemented in PLINK2 for SNPs with minor allele frequency (MAF) ≥ 0.01 and imputation quality score (R2) > 0.5 [28]. In family-based designs, cases and pseudocontrols are matched on environmental variables and genetic ancestry, eliminating the need for additional covariates. For population-based studies, covariates such as principal components are included to account for population stratification. Meta-analysis with previous GWAS datasets is performed using tools like METAL to enhance statistical power [28].
Figure 1: GWAS Workflow for ASD Genetics. The diagram illustrates the sequential steps from sample collection to functional validation of findings.
Whole Exome/Genome Sequencing Analysis
For sequencing-based studies, the analytical pipeline begins with quality assessment of raw sequencing data, followed by alignment to a reference genome (typically GRCh38 in recent studies) [23]. Variant calling employs multiple callers such as GATK and DeepVariant to enhance sensitivity and specificity [24]. Joint calling across samples improves accuracy for low-frequency variants [23]. Annotation of variants incorporates multiple databases to predict functional consequences, including effects on protein coding, regulatory elements, and evolutionary conservation.
Rare variant association tests for ASD typically employ specialized statistical frameworks like the transmission and de novo association (TADA) test, which integrates multiple lines of evidence including de novo mutations, rare inherited variants, and case-control differences in mutation burden [23]. The TADA+ framework, developed by the Autism Sequencing Consortium, incorporates data from tens of thousands of trios to identify genes with FDR < 0.1 [23]. For structural variant detection, multiple algorithms are often combined, followed by manual curation to reduce false positives.
Network Analysis and Integration Approaches
Network analysis of ASD genetic data involves constructing protein-protein interaction (PPI) networks using databases like STRING (confidence score threshold ≥ 0.4) and importing into visualization software such as Cytoscape [26]. Differential expression analysis identifies significantly up- and down-regulated genes using linear modeling approaches with thresholds of |log2FC| > 1.5 and adjusted p-value (FDR) < 0.05 [26]. Functional enrichment analysis employing Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways uses hypergeometric distribution with multiple testing correction [26].
Network-based drug prediction utilizes the Connectivity Map (CMap) platform to identify potential therapeutic compounds that reverse expression signatures associated with ASD [26]. Immune infiltration correlation analysis explores associations between key genes and immune cell subpopulations using deconvolution algorithms implemented in R packages like "GSVA" [26]. Machine learning approaches, particularly random forest classifiers, are employed to identify feature genes with the highest importance scores for autism prediction, with performance validation through out-of-bag error estimates and receiver operating characteristic (ROC) analysis [26].
Figure 2: Network Analysis Workflow. The process integrates multiple data types to identify key genes and pathways in ASD.
Genomic Datasets and Consortia
SPARK (Simons Foundation Powering Autism Research for Knowledge): Provides genomic data from over 115,000 participants, including WES data from 44,000+ individuals with autism. The resource includes mapped sequencing reads, SNP array genotyping data, and variant call files from multiple callers [24]. Access is controlled by a Data Access Committee for approved researchers.
MSSNG: A whole-genome sequencing resource containing data from 11,312 individuals (5,100 with ASD) aligned to GRCh38. The dataset includes joint-called small variants, structural variant calls, tandem repeat expansions, and polygenic risk scores [23]. Data are stored on the Google Cloud Platform with variant calls, annotations, and phenotype data available as BigQuery tables.
Autism Sequencing Consortium (ASC): An international collaboration that shares ASD samples and genetic data, currently working to sequence more than 50,000 exomes. The ASC hosts shared data and analysis at a single site to enable joint analysis of large-scale data from many groups [25].
Simons Simplex Collection (SSC): Includes permanently available genetic and phenotypic data from 2,600 simplex families (families with one child affected by ASD and unaffected parents and siblings). The collection includes detailed phenotypic assessments and serves as a valuable replication cohort [3].
Analytical Tools and Software
PLINK: A whole-genome association analysis toolset used for quality control, association analysis, and population stratification analysis. Essential for GWAS preprocessing and analysis [28].
STRENGTH (Structural Variation Detection): Tools for identifying copy number variants and other structural variations from WGS data. Critical for comprehensive variant detection beyond SNVs [23].
Cytoscape: An open-source platform for complex network visualization and analysis. Used for constructing and analyzing protein-protein interaction networks in ASD [26].
TADA (Transmission And De Novo Association): A statistical framework for identifying disease-associated genes by integrating de novo mutations and rare inherited variants. The enhanced TADA+ version incorporates data from tens of thousands of trios [23].
Table 3: Essential Research Reagents and Resources
| Resource Type | Specific Examples | Application in ASD Research |
|---|---|---|
| Genomic Datasets | SPARK, MSSNG, SSC, ASC [24] [23] [25] | Primary data sources for genetic discovery and validation |
| Analysis Tools | PLINK, EAGLE, METAL, TADA [28] [23] | Quality control, association testing, meta-analysis, rare variant association |
| Network Analysis | Cytoscape, STRING database, clusterProfiler [26] | PPI network construction, functional enrichment analysis |
| Functional Validation | Massively Parallel Reporter Assays (MPRA) [28] | Experimental validation of non-coding variant function |
| Expression Data | GTEx, BrainSpan, GEO datasets (e.g., GSE18123) [26] | Context-specific gene expression patterns and eQTL mapping |
The integration of large-scale genomic resources has fundamentally transformed our understanding of ASD genetics, moving from isolated discoveries to systematic mapping of risk genes and biological pathways. The convergence of findings from GWAS, WES, and WGS approaches has revealed a complex genetic architecture encompassing common and rare variants, coding and non-coding regions, and diverse molecular mechanisms. Network-based analyses have demonstrated that apparently heterogeneous genetic risk factors converge on coherent biological pathways, particularly those involved in synaptic function, chromatin modification, and transcriptional regulation.
Future research will need to address several key challenges. First, increasing ancestral diversity in ASD genomic studies remains imperative, as current resources are still predominantly European-ancestry individuals. Second, integrating multi-omic data—including epigenomic, transcriptomic, and proteomic profiles—will provide a more comprehensive view of the molecular mechanisms underlying ASD. Third, bridging the gap between genetic discovery and clinical application requires improved understanding of genotype-phenotype relationships and developmental trajectories, as exemplified by recent work decomposing phenotypic heterogeneity and identifying genetic programs underlying clinical differences [3] [4]. As these efforts mature, they hold promise for developing targeted interventions based on an individual's specific genetic profile and ultimately improving outcomes for autistic individuals across the lifespan.
Computational Methods for Uncovering Network Topology and Core Genes in ASD
Network propagation has emerged as a powerful computational technique for integrating multi-omic data within the context of protein-protein interaction (PPI) networks, offering significant potential for identifying autism spectrum disorder (ASD) risk genes. This method functions by simulating the flow of information through a biological network, starting with seed genes and propagating their influence to nearby nodes, thereby prioritizing genes based on their network proximity to known ASD-associated genes. By leveraging this approach on a scaffold of documented protein interactions, researchers can effectively combine diverse genomic, transcriptomic, and proteomic datasets. This integration provides a more comprehensive understanding of the complex molecular interactions underlying ASD, ultimately helping to pinpoint high-confidence candidate genes and biological pathways for further experimental validation and therapeutic targeting [30] [31].
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental condition affecting an estimated 1 in 44 children, characterized by challenges in social communication, behavior, and learning. Its etiology is profoundly heterogeneous, involving a complicated interplay of genetic and environmental factors. A critical step in unraveling ASD's pathophysiology is identifying its genetic underpinnings, which is challenging due to the disorder's polygenic nature and the modest effect sizes of many contributing variants [30]. Extensive molecular studies have charted the ASD landscape across various information layers, including genome-wide association studies (GWAS), differential gene expression, alternative splicing changes, differential methylation, and copy number variations. Each investigation typically generates candidate lists of ASD-associated genes, creating a pressing need for computational methods that can consolidate these findings into a unified framework [30].
Network propagation offers a robust solution to this integration challenge. This method is grounded in the concept that genes associated with a specific disorder are not randomly distributed in a biological network but tend to cluster together or reside in specific network neighborhoods. The technique involves simulating a "random walk" on a PPI network, where known disease-associated genes serve as seeds. The influence of these seeds is then propagated to adjacent nodes, assigning a score to every gene in the network that reflects its proximity to the seed set. This approach effectively smooths noisy omics data and leverages the local structure of the interactome to prioritize candidate genes based on their network relationships rather than just individual statistical significance [30] [31]. Within the context of ASD, this is particularly valuable for discovering genes that may not reach genome-wide significance in standalone studies but that reside in network modules densely populated with other ASD risk genes, suggesting their functional relevance.
Core Methodology: A Technical Deep Dive
Data Integration and Feature Generation
The first stage in constructing a network propagation model for ASD involves the careful curation of diverse omic data sets to be used as seeds for the propagation process. The goal is to capture the multifaceted molecular perturbations associated with the disorder.
Table 1: Exemplary Multi-Omic Data Sources for ASD Network Propagation
| Data Type | Data Source | Number of Genes | Biological Insight |
|---|---|---|---|
| Differential Gene Expression (DGE) | Cortex Samples [30] | 1,611 | Identifies genes with altered mRNA levels in post-mortem ASD brain tissues. |
| Differential Alternative Splicing | Cortex Samples [30] | 833 | Pinpoints genes with disrupted RNA splicing patterns in ASD. |
| Transmitted/De Novo Association (TADA) | Whole-Exome Sequencing [30] | 102 | Highlights genes enriched for rare, high-impact genetic variants. |
| Differential DNA Methylation & Expression | Cross-Cortex Analysis [30] | 18 | Reveals genes whose regulation may be affected by epigenetic changes. |
| Analysis of De Novo CNVs | Simons Simplex Collection [30] | 65 | Identifies genes located in genomic regions affected by copy number variations. |
Each of these ASD-related gene lists is used as a seed for an independent network propagation process on a PPI network. The human PPI network from Signorini et al. (2021), comprising 20,933 proteins and 251,078 interactions in its main connected component, serves as an effective scaffold. The initial value of each seed protein from a list of size s is set to 1/s. Network propagation is then run with a damping parameter ɑ = 0.8, a common setting that balances the influence of the seed nodes with the global network structure. The results are normalized using the eigenvector centrality method to prevent biases arising from the varying degrees (connectedness) of proteins within the network. The output is a set of propagation scores for each gene, representing its network proximity to the seed genes from each distinct omic dataset. These scores become the feature set for the gene in subsequent predictive modeling [30].
Machine Learning Integration
Once network-propagated features are generated for each gene, they are integrated using a machine learning model to produce a unified prediction score for ASD association. A random forest classifier is frequently employed for this task due to its ability to handle high-dimensional data and model complex interactions between features without overfitting.
The model requires a set of positive and negative examples for training. The SFARI Gene Scoring Module is a standard resource for this, providing a expert-curated assessment of evidence for gene association with ASD. In a typical training setup, "Category 1" (High Confidence) genes from SFARI are used as positives. An equal number of negative genes are randomly selected from those not present in the SFARI database to create a balanced dataset. The random forest model is then trained using the propagated feature vectors of these genes. A common implementation uses the sklearn Python package with default parameters: a maximum of 100 trees, no maximum tree depth, and a minimum of 2 samples required to split an internal node. The model's performance is rigorously evaluated using 5-fold cross-validation, assessing metrics like the Area Under the Receiver Operating Characteristic Curve (AUROC) and the Area Under the Precision-Recall Curve (AUPRC) [30].
Experimental Protocol and Validation
Performance Benchmarking
To validate the efficacy of the network propagation approach, its performance must be compared against existing state-of-the-art methods. A recent study demonstrated that a model integrating ten propagated features achieved a mean AUROC of 0.87 and an AUPRC of 0.89 in cross-validation, indicating high predictive accuracy [30]. Furthermore, this integrated model was shown to outperform the previous leading predictor, forecASD. When the same random forest classifier was trained on the features used by forecASD (BrainSpan expression and STRING interaction data), it yielded an AUROC of 0.87. In contrast, the network propagation features achieved a superior AUROC of 0.91 on the same dataset. As a negative control, running the propagation procedure on a random degree-preserving network still resulted in a relatively high AUROC (0.82), which underscores the intrinsic quality of the seed gene sets but also highlights the critical importance of using a biologically accurate PPI network [30].
Table 2: Model Performance Comparison (AUROC)
| Method | Data Features | AUROC |
|---|---|---|
| Integrated Network Propagation | 10 propagated scores from multi-omic data [30] | 0.91 |
| forecASD Benchmark | BrainSpan expression & STRING network [30] | 0.87 |
| Negative Control | Propagation on random degree-preserving network [30] | 0.82 |
After establishing model accuracy, an optimal classification cutoff (e.g., 0.86) can be calculated to maximize the product of specificity and sensitivity, facilitating the application of the model to predict novel ASD-associated genes. The biological relevance of the top-predicted genes is further confirmed by showing that their scores are significantly higher than those of random negative genes for SFARI categories 2 and 3, which were not used in the initial model training [30].
Functional Enrichment Analysis
Prioritized gene lists must be interpreted through the lens of biological function. Functional enrichment analysis of the top 84 genes predicted by a network propagation model (using a threshold that maximizes the sum of precision and recall) revealed several key pathways and phenotypes associated with ASD.
From the Human Phenotype Ontology, "Autistic Behavior" was the most significantly enriched term. Gene Ontology analysis of Biological Processes (GO:BP) and Molecular Functions (GO:MF) further connected these top genes to critical neural functions, providing a biological sanity check for the computational predictions and suggesting potential mechanisms for the disorder's pathophysiology [30]. This step is crucial for transitioning from a list of candidate genes to actionable biological insights.
The Scientist's Toolkit: Essential Research Reagents
Implementing a network propagation pipeline for ASD gene discovery requires a suite of key data resources and software tools.
Table 3: Key Research Reagents and Resources
| Resource Name | Type | Function in Analysis |
|---|---|---|
| SFARI Gene Database [30] | Gene Database | Provides expert-curated gene scores used as gold-standard labels for training and validating predictive models. |
| STRING Database [32] | PPI Network | A source of protein-protein interaction data that can serve as the scaffold for network propagation algorithms. |
| Signorini PPI Network [30] | PPI Network | A high-quality, manually curated human PPI network used specifically in the referenced study. |
| Cytoscape & CytoHubba [31] [32] | Network Analysis Software | Platforms for visualizing complex molecular interaction networks and identifying highly connected hub genes. |
| g:Profiler [30] | Functional Enrichment Tool | Used to perform statistical enrichment analysis of gene lists against GO terms, pathways, and phenotypic ontologies. |
| sklearn (Scikit-learn) [30] | Python Library | Provides the machine learning framework (e.g., Random Forest classifier) for integrating features and making predictions. |
Signaling Pathways and Convergent Biology
Network propagation studies in ASD have consistently revealed a convergent molecular architecture, implicating specific biological pathways and processes. A prominent finding is the convergence of multiple ASD-linked transcriptional regulators on a common set of synaptic genes. Research involving the depletion of nine different ASD-risk transcriptional regulators (including chromatin modifiers like CHD8 and SETD5, and transcription factors like TBR1) in primary neurons showed that despite their disparate primary functions, they disrupt the expression of a shared set of genes encoding critical synaptic proteins. This convergence was further reflected in a drastic disruption of neuronal firing patterns throughout maturation, linking transcriptional dysregulation directly to aberrant neuronal function [33].
Furthermore, a convergent molecular network has been identified underlying both ASD and congenital heart disease (CHD), explaining the clinical co-morbidity between these conditions. Network genetics approaches have pinpointed 101 genes with shared genetic risk for both disorders. This shared network is highly enriched for genes involved in specific pathways, with a family of ion channels (e.g., the sodium transporter SCN2A) being a key convergent pathway linking these functions to early brain and heart development. Validation in model systems like Xenopus tropicalis confirmed that disruption of these shared risk genes causes abnormalities in both organ systems [34].
Beyond the genome and transcriptome, phosphoproteomic studies add another layer of regulation. Multi-omics investigations of ASD mouse models (Shank3Δ4–22 and Cntnap2−/−) have identified that autophagy-related pathways are particularly affected. While global proteomics showed changes in postsynaptic components and mTOR signaling, phosphoproteomics revealed unique phosphorylation sites in key autophagy-related proteins like ULK2, RB1CC1, and ATG16L1. This suggests that altered phosphorylation patterns, not just expression changes, contribute significantly to the impaired autophagic flux observed in ASD models, highlighting a potential post-translational mechanism in the disorder's pathology [35].
Network propagation represents a paradigm shift in how researchers integrate complex, multi-scale biological data to elucidate the foundations of polygenic disorders like ASD. By using a PPI network as a scaffold, this method provides a powerful framework for consolidating evidence from genomic, transcriptomic, and proteomic studies, effectively prioritizing high-confidence candidate genes that reside in relevant biological neighborhoods. The consistent discovery of convergent pathways—such as synaptic gene regulation, ion channel function, and autophagy—through independent network-based analyses underscores the robustness of this approach. As PPI networks become more complete and multi-omic datasets continue to expand, network propagation will remain an indispensable tool for translating genetic associations into a functional understanding of ASD biology, ultimately guiding the development of novel therapeutic strategies.
Autism Spectrum Disorder (ASD) is characterized by profound genetic heterogeneity, involving hundreds of risk genes that converge on a limited set of neurodevelopmental pathways. Weighted Gene Co-Expression Network Analysis (WGCNA) has emerged as a powerful systems biology approach to navigate this complexity by identifying modules of highly correlated genes in transcriptomic data, revealing functional networks dysregulated in ASD. Unlike differential expression analysis that focuses on individual genes, WGCNA considers the network topology of the entire transcriptome, capturing subtle but coordinated changes across biological pathways. This approach has proven particularly valuable for elucidating the molecular mechanisms underlying ASD, as functionally related genes often exhibit coordinated expression patterns despite diverse genetic origins. Research demonstrates that ASD risk genes systematically coalesce into co-expression modules enriched for specific biological functions during human cortical development, including synaptic formation, chromatin remodeling, and immune responses [36] [37] [38]. By mapping these networks, researchers can identify central "hub genes" that may exert disproportionate influence on biological processes and represent promising targets for therapeutic intervention.
Key Dysregulated Modules and Pathways in ASD
WGCNA studies of postmortem ASD brain tissues have consistently identified specific dysregulated modules that illuminate the disorder's pathophysiology. These modules reflect core disruptions in neuronal function, immune processes, and cortical patterning.
Table 1: Key Dysregulated Co-Expression Modules Identified in ASD Brain Transcriptomes
| Module/Study | Expression in ASD | Key Functions/Pathways | Notable Hub Genes | Cellular Context |
|---|---|---|---|---|
| Neuronal Module (M12) [36] | Downregulated | Synaptic transmission, vesicular transport | A2BP1, APBA2, SCAMP5, CNTNAP1 | Neurons, especially inhibitory neurons |
| Immune/Glial Module (M16) [36] | Upregulated | Immune/inflammatory response, astrocyte/microglia markers | - | Astrocytes, activated microglia |
| M2-Microglial Module (mod5) [37] | Upregulated | Type I interferon pathway, cytokine signaling, M2 microglial state | - | M2-activated microglia |
| Neuronal Module (mod1) [37] | Downregulated | Synaptic transmission, neuronal development | - | Neurons |
| Histone Module [7] | Dysregulated | Histone modification, neuronal differentiation | - | Neural progenitor cells, neurons |
Beyond these consistent module alterations, WGCNA has revealed fundamental disruptions in cortical organization in ASD. Remarkably, regional transcriptomic signatures that typically distinguish frontal and temporal cortex are significantly attenuated in ASD brains, suggesting abnormalities in cortical patterning established during fetal development [36]. This finding aligns with anatomical evidence of reduced structural differentiation between cortical regions in ASD, implicating impaired regional specification as a core disease mechanism.
Recent single-cell transcriptomic analyses further refine our understanding of which neuronal populations are most vulnerable in ASD. Multiple independent studies indicate that ASD risk genes show enriched expression in inhibitory neurons, and their downstream targets are similarly enriched in these populations, suggesting inhibitory neurons may be a major affected cell type [39]. This provides molecular evidence supporting the long-standing excitatory/inhibitory (E/I) imbalance hypothesis of ASD pathophysiology.
Experimental Framework and Methodological Protocols
Implementing WGCNA requires careful experimental design and computational execution. The following workflow outlines the key stages for conducting a comprehensive WGCNA study of brain transcriptomes in ASD.
Sample Preparation and RNA Sequencing
The initial stage involves careful sample acquisition and processing. Studies typically utilize postmortem brain tissues from ASD individuals and matched controls, focusing on cortical regions implicated in ASD such as prefrontal cortex (BA9) and superior temporal gyrus (BA41/42) [36]. The RNA integrity number (RIN) should be confirmed to exceed 7.0, with no significant differences in age, post-mortem interval, or RIN between case and control groups [36]. For sequencing, libraries are prepared using standardized protocols (e.g., Illumina TruSeq), sequenced to appropriate depth (typically 30-50 million reads per sample), and aligned to the reference genome (e.g., GRCh37/hg19) using aligners like STAR [39].
WGCNA Implementation and Parameters
The core WGCNA procedure involves constructing co-expression networks and identifying modules:
Data Preprocessing: Filter lowly expressed genes and remove outliers using the
goodSamplesGenesfunction in WGCNA R package. Normalize expression data and adjust for technical covariates.Network Construction: Select an appropriate soft-thresholding power (β) using the
pickSoftThresholdfunction to achieve scale-free topology fit (typically R² > 0.8-0.9) [7] [36]. For brain transcriptome data, powers of 12-18 are commonly employed [39].Module Detection: Identify modules using the
blockwiseModulesfunction with a minimum module size of 30 genes [7]. Merge highly correlated modules (typically |correlation| > 0.9) [7]. Calculate module eigengenes (MEs) as the first principal component of each module.Module-Phenotype Association: Correlate MEs with ASD status using linear models, adjusting for covariates like age, sex, and batch effects. Modules with eigengenes significantly associated with ASD status (FDR < 0.05) are selected for downstream analysis.
Downstream Bioinformatics Analyses
Following module identification, several analytical steps extract biological insights:
Functional Enrichment: Perform Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment using tools like
clusterProfiler[7] [26]. Significant terms (FDR < 0.05) reveal biological processes dysregulated in ASD.Hub Gene Identification: Calculate module membership (MM) as the correlation between gene expression and module eigengene. Genes with high MM (typically > 0.8-0.9) are considered hub genes [7] [36].
Integration with Genetic Risk Data: Overlap module genes with known ASD risk genes from databases like SFARI [36] [39]. This determines whether genetically associated genes converge on specific co-expression modules.
Cross-Study Validation: Validate findings in independent datasets and experimental models, such as human cerebral organoids or neuronal cultures [7] [39].
Integration with Genetic and Phenotypic Data
Advanced applications of WGCNA extend beyond transcriptomic analysis to integrate genetic and clinical data, revealing how dysregulated networks align with genetic risk and phenotypic heterogeneity.
Recent studies have demonstrated that the neuronal module enriched for ASD risk genes shows significant enrichment for genetically associated variants, providing independent support for the causal involvement of these genes in autism [36]. In contrast, the immune-glial module typically shows no such enrichment, suggesting non-genetic or environmental etiology for this aspect of ASD pathophysiology [36].
A landmark 2025 study decomposed ASD phenotypic heterogeneity into four robust classes using generative mixture modeling of 239 phenotypic features across 5,392 individuals [3]. These classes—Social/behavioral, Mixed ASD with developmental delay, Moderate challenges, and Broadly affected—demonstrated distinct genetic architectures:
Table 2: Phenotypic Classes and Their Genetic Correlates in ASD
| Phenotypic Class | Sample Size | Core Features | Genetic Correlates |
|---|---|---|---|
| Social/behavioral | 1,976 | High social communication deficits, disruptive behavior, attention deficit | Distinct patterns in common genetic variation measured by polygenic scores |
| Mixed ASD with DD | 1,002 | Nuanced ASD features with strong developmental delays | Enriched for rare inherited variation affecting different pathways |
| Moderate challenges | 1,860 | Consistently lower scores across all difficulty categories | Distinct common variant profiles |
| Broadly affected | 554 | High scores across all measured difficulty categories | Earlier age of diagnosis, associated with specific genetic programs |
WGCNA further revealed that class-specific differences in the developmental timing of affected genes align with clinical outcome differences, with earlier-expressed gene sets disrupted in classes with more pronounced developmental delays [3]. This person-centered approach captures how combinations of traits manifest in individuals rather than analyzing traits in isolation, offering stronger clinical value for prognosis.
Research Reagent Solutions Toolkit
Table 3: Essential Research Reagents and Computational Tools for WGCNA Studies
| Tool/Reagent | Specific Function | Application in ASD WGCNA Studies |
|---|---|---|
| WGCNA R Package [36] [39] | Construction of weighted co-expression networks | Primary tool for identifying gene modules from transcriptomic data |
| STRING Database [7] [26] | Protein-protein interaction network generation | Validating physical interactions between dysregulated proteins |
| Cytoscape [7] | Network visualization and analysis | Visualizing co-expression networks and identifying subnetworks |
| clusterProfiler [7] | Functional enrichment analysis | Identifying overrepresented GO terms and KEGG pathways in modules |
| Seurat [7] | Single-cell RNA-seq analysis | Validating cell-type specificity of hub genes at single-cell resolution |
| SFARI Gene Database [39] [37] | Curated ASD risk genes | Determining enrichment of known ASD genes in identified modules |
| Human Brain Tissue Banks | Source of postmortem brain tissues | Providing region-matched ASD and control brain samples for RNA-seq |
| Cerebral Organoids [7] [39] | 3D in vitro model of human brain development | Validating functional role of hub genes in human neural development |
| Primary Neuronal Cultures [33] | Simplified in vitro neuronal system | Testing effects of ASD gene disruption on neuronal firing and gene expression |
Signaling Pathways and Molecular Mechanisms
WGCNA studies have illuminated several key signaling pathways disrupted in ASD, with two particularly prominent circuits emerging: the immune-microglial and synaptic-neuronal pathways. The interplay between these systems represents a crucial mechanism in ASD pathophysiology.
The molecular pathways illustrated above reflect convergent findings from multiple WGCNA studies. Research on Pitt-Hopkins syndrome (PTHS), a monogenic form of ASD caused by TCF4 mutations, revealed that hub genes in dysregulated modules include those encoding proteins involved in histone modification, synaptic vesicle trafficking, and cell signaling [7]. This suggests that transcriptional regulators connected to ASD converge on specific downstream targets, particularly synaptic genes.
Experimental validation studies depleting nine ASD-linked transcriptional regulators in primary neurons found shared gene expression signatures converging on synaptic genes, with corresponding disruptions to neuronal firing patterns throughout maturation [33]. This demonstrates that despite diverse molecular functions, ASD risk genes produce convergent functional effects on neuronal activity, potentially underlying core behavioral symptoms.
WGCNA has fundamentally advanced our understanding of ASD pathophysiology by revealing how hundreds of genetically heterogeneous risk genes converge on discrete co-expression networks governing neurodevelopment. The consistent identification of neuronal, immune, and cortical patterning modules across independent studies provides a robust framework for understanding ASD's molecular architecture.
Future research directions will likely focus on several promising areas: First, integrating WGCNA with single-cell and spatial transcriptomics will resolve network dysregulation at cellular resolution within intact tissue contexts. Second, longitudinal WGCNA across developmental timepoints will capture dynamic network alterations during critical periods. Third, combining WGCNA with functional genomics in human cellular models will establish causal relationships between genetic variation, network disruption, and cellular phenotypes. Finally, translating these findings into clinically useful biomarkers and therapeutic targets represents the ultimate frontier, with recent studies already identifying potential small-molecule interventions based on network reversal predictions [26].
As cohort sizes increase and analytical methods refine, WGCNA will continue to illuminate the complex interplay between genetic risk, transcriptional regulation, and clinical manifestation in ASD, ultimately paving the way for personalized interventions based on an individual's specific network pathology profile.
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental condition characterized by remarkable genetic and clinical heterogeneity. Research indicates that over 1200 genes have been associated with ASD risk, yet no single gene accounts for more than 1-2% of cases [40]. This diversity suggests that ASD pathogenesis arises from disruptions in interconnected biological networks rather than isolated genetic defects. The emerging paradigm in ASD research therefore focuses on identifying hub genes—highly connected central players within molecular networks—that may orchestrate convergent pathological pathways despite genetic heterogeneity.
Network-based analyses reveal that the genetic architecture of ASD converges on specific biological processes and brain cell types [40]. Hub genes occupy critical positions within protein-protein interaction (PPI) networks and gene co-expression modules, making them potential master regulators of ASD-associated pathophysiology. Their central positioning means that perturbations to these hubs can impart disproportionately widespread disturbances to overall network function [41]. Identifying these pivotal elements provides a strategic approach to deciphering ASD's complex etiology and uncovering novel therapeutic targets that address core biological mechanisms rather than superficial symptomatic manifestations.
Table 1: Key Concepts in ASD Network Topology
| Concept | Definition | Research Implication |
|---|---|---|
| Hub Genes | Highly connected nodes within biological networks | Potential master regulators of convergent pathways |
| Network Modules | Groups of strongly interconnected genes/proteins | Represent functional biological units disrupted in ASD |
| Rich-Club Organization | Tendency of high-degree hubs to interconnect | Explains disproportionate impact of hub perturbations |
| Gene Co-expression | Correlation in expression patterns across samples | Identifies functionally related gene sets |
Methodological Approaches for Hub Gene Identification
Network Construction and Module Detection
The initial step in hub gene identification involves reconstructing biological networks from molecular data. Weighted Gene Co-expression Network Analysis (WGCNA) represents one established methodology for identifying modules of highly correlated genes [42]. This approach constructs a weighted network where connection strengths between genes are determined by the absolute value of the correlation coefficient between their expression profiles, typically raised to a power β (soft-thresholding parameter) to emphasize strong correlations.
For higher-resolution network partitioning, the Leiden community detection algorithm offers advanced capabilities for identifying stable gene communities within complex co-expression networks [43]. The hierarchical application of this algorithm enables researchers to progressively refine network modules, enhancing biological interpretability. The construction of these networks begins with calculating all pairwise Pearson correlations between gene expression profiles, retaining only statistically significant connections (e.g., at 99% confidence interval) to reduce noise [43].
Hub Gene Selection within Modules
Once network modules are identified, several metrics can prioritize hub genes within each module. Module membership (also known as eigengene-based connectivity, kME) quantifies how closely a gene's expression pattern correlates with the module's summary expression profile (module eigengene). Genes with high kME values are considered intramodular hubs strongly representative of the module's biological function.
Alternatively, network theoretic measures including degree centrality (number of connections), betweenness centrality (frequency as shortest path), and closeness centrality (average distance to other nodes) can identify topologically central genes. In practice, researchers often combine these approaches, selecting genes that rank highly for both intramodular connectivity and statistical significance in differential expression analyses [26] [43].
Table 2: Comparison of Hub Gene Identification Methods
| Method | Key Features | Advantages | Limitations |
|---|---|---|---|
| WGCNA | Identifies modules via hierarchical clustering; uses soft thresholding to preserve continuous correlation information | Preserves biological nuance; robust to noise; establishes module-trait associations | Computationally intensive for large datasets; parameter selection influences results |
| Leiden Algorithm | Advanced community detection optimizing partition quality; hierarchical implementation | Higher resolution than traditional methods; identifies stable communities; handles large networks effectively | Complex implementation; requires multiple random initializations for stability assessment |
| PPI Network Analysis | Constructs networks of physical protein interactions from existing databases (STRING) | Leverages curated biological knowledge; identifies physically interacting hubs | Limited to proteins with established interactions; database coverage varies |
Key Signaling Pathways and Biological Processes
Hub genes in ASD consistently converge on several key biological pathways despite the disorder's genetic heterogeneity. Functional enrichment analyses repeatedly identify disruptions in synaptic function, neuronal development, immune regulation, and chromatin remodeling as central pathological mechanisms [26] [40] [9].
Synaptic pathways are particularly prominent, with hub genes frequently involved in neurotransmitter signaling (GABA and glutamate systems), synaptic scaffolding (SHANK family proteins), and neuronal connectivity [9]. The significant enrichment of hub genes in subplate and intermediate zones during mid-gestation highlights the importance of early cortical development, suggesting that disruptions to initial brain network establishment underlie ASD pathophysiology [41]. Immune and inflammatory pathways also feature prominently, with hub genes associated with microglial function and neuroimmune interactions appearing consistently across multiple studies [26] [40].
Diagram 1: Hub Genes Converge on Key Biological Pathways in ASD. Central hub genes (colored by functional category) influence distinct but interconnected biological processes that collectively contribute to neural circuit dysfunction and core ASD symptoms.
Experimental Protocols and Workflows
Integrated Protocol for Hub Gene Identification
A comprehensive approach to hub gene identification combines multiple analytical methods to leverage their complementary strengths. The following integrated protocol has demonstrated success in recent ASD studies [26] [42] [43]:
Step 1: Data Acquisition and Preprocessing
- Obtain transcriptomic data from relevant databases (e.g., GEO accession GSE18123 for ASD)
- Perform quality control, background correction, and normalization using packages like limma in R
- Address batch effects using established methods (e.g., ComBat from the sva package)
Step 2: Differential Expression Analysis
- Identify differentially expressed genes (DEGs) using linear models (limma package)
- Apply thresholds (e.g., |log2FC| > 0.585 and adjusted p-value < 0.05) to select significant DEGs
Step 3: Network Construction and Module Detection
- Construct co-expression networks using WGCNA or correlation-based approaches
- Identify modules of co-expressed genes through hierarchical clustering or Leiden algorithm
- Correlate module eigengenes with clinical traits to identify biologically relevant modules
Step 4: Hub Gene Selection
- Calculate module membership (kME) for all genes within significant modules
- Compute network centrality measures (degree, betweenness) within modules
- Prioritize genes with high intramodular connectivity and statistical significance
Step 5: Validation and Functional Characterization
- Validate hub genes in independent datasets (e.g., GSE28521)
- Perform functional enrichment analysis (GO, KEGG) on hub genes and their modules
- Conduct immune infiltration correlation analysis if relevant to disease context
Diagram 2: Integrated Workflow for Hub Gene Identification. The process flows sequentially from data acquisition through network analysis to final hub gene identification and validation, with each stage building upon the previous one.
Table 3: Essential Research Resources for Hub Gene Identification Studies
| Resource Category | Specific Examples | Function/Purpose |
|---|---|---|
| Data Resources | GEO datasets (GSE18123, GSE28475, GSE28521) | Provide transcriptomic data for analysis |
| Bioinformatics Tools | Limma R package, WGCNA, Leiden algorithm | Perform differential expression, network construction, module detection |
| Network Databases | STRING database, GeneMANIA | Construct protein-protein interaction networks |
| Functional Annotation | clusterProfiler, Enrichr | Conduct GO, KEGG pathway enrichment analysis |
| Validation Resources | CMap database, SFARI Gene database | Predict drug candidates, access curated ASD gene sets |
Case Studies and Research Applications
Validated ASD Hub Genes and Their Clinical Implications
Several hub genes have been consistently identified across multiple ASD studies, providing compelling evidence for their central roles in disease pathophysiology. Recent research applying random forest analysis to transcriptomic data identified ten key feature genes with the highest importance scores for autism prediction: SHANK3, NLRP3, SERAC1, TUBB2A, MGAT4C, TFAP2A, EVC, GABRE, TRAK1, and GPR161 [26]. Among these, MGAT4C demonstrated particularly strong discriminatory power (AUC = 0.730) in receiver operating characteristic analysis, highlighting its potential as a robust diagnostic biomarker [26].
Another study investigating the comorbidity between ASD and sleep disturbances identified LAMC3 as a crucial shared hub gene [42]. This gene plays a vital role in neural development and is associated with cortical malformations. The study further constructed a miRNA-LAMC3 regulatory network, highlighting hsa-miR-140-3p as a potential key regulator of LAMC3 expression [42]. Immune infiltration analyses in this study revealed significant correlations between LAMC3 expression and specific immune cell populations, suggesting interconnected neuroimmune mechanisms in ASD pathogenesis.
From Hub Genes to Therapeutic Candidates
The identification of hub genes creates opportunities for developing targeted interventions. Connectivity Map (CMap) analysis has proven valuable for predicting potential therapeutic compounds that might reverse ASD-associated gene expression signatures [26]. This approach has identified candidate drugs consistent with some clinical trial results, supporting its predictive validity.
Promisingly, hub gene identification is already informing drug development pipelines. Several companies are pursuing therapies targeting specific ASD-related pathways: Stalicla SA is developing STP1 for the ASD-Phen1 subgroup; MapLight Therapeutics has ML-004 targeting glutamatergic and GABAergic signaling; Roche is advancing RO7017773 as a selective GABAA α5 receptor modulator; and Yamo Pharmaceuticals is investigating L1-79 as a monoamine modulator [44]. These approaches represent a shift from symptomatic management toward targeting core biological mechanisms identified through network-based analyses.
Hub gene identification represents a powerful paradigm for deciphering the complex molecular architecture of ASD. By focusing on central players within biological networks, researchers can transcend the limitations of studying individual risk genes and instead target coordinated functional modules. The consistent convergence of hub genes on specific biological pathways—particularly synaptic function, neuronal development, and immune regulation—provides a mechanistic foundation for understanding ASD heterogeneity while revealing points of commonality across genetically distinct cases.
Future research directions will likely emphasize single-cell resolution analyses to identify hub genes within specific neural cell types, integration of multi-omics data to capture different layers of regulation, and application of advanced machine learning methods to predict network perturbations. The ongoing refinement of community detection algorithms and validation approaches will further enhance our ability to distinguish true biological hubs from methodological artifacts. As these techniques mature, hub gene identification promises to deliver not only deeper insights into ASD pathogenesis but also novel biomarkers for early detection and personalized intervention strategies tailored to an individual's specific network pathology.
The Connectivity Map (CMap) is a powerful resource created by the Broad Institute to enable a systematic, data-driven approach to understanding human disease and accelerating drug discovery [45]. Its core hypothesis is that a comprehensive catalog of cellular signatures from genetic and pharmacologic perturbations can serve as a functional look-up table of the genome, revealing previously unrecognized connections between proteins operating in the same pathway, between small molecules and their protein targets, or between structurally dissimilar compounds with similar functions [45]. This approach has gained significant attention for drug repurposing because it potentially overcomes bottleneck constraints faced by traditional drug discovery in terms of cost, time, and risk [46].
For researchers investigating Autism Spectrum Disorder (ASD), CMap offers a promising computational framework to bridge the gap between genetic findings and therapeutic interventions. ASD is characterized by complex genetic heterogeneity, involving disruptions in multiple genes and pathways [14]. Network-based analyses of ASD have revealed that the condition involves dysregulation of genes controlling both neurological and metabolic functions, suggesting that systematic approaches like CMap could identify compounds that reverse these aberrant expression patterns [14]. By treating ASD-associated gene expression signatures as queries against the CMap database, researchers can rapidly identify candidate compounds that may reverse these pathological signatures, potentially leading to new treatment strategies.
Understanding the CMap Framework and Technological Infrastructure
Core Principles and Data Generation
The fundamental principle behind CMap is the concept of "connectivity" - the idea that perturbations causing similar changes in gene expression likely target related biological pathways [45]. To produce data at the required scale, CMap employs the L1000 technology, a relatively inexpensive and rapid high-throughput gene expression profiling method that directly measures the expression of 978 "landmark" genes and computationally infers the expression of another 11,350 genes [45]. This approach has enabled the generation of a massive library containing over 1.5 million gene expression profiles from approximately 5,000 small-molecule compounds and 3,000 genetic reagents tested across multiple cell types [45].
The CMap database structure is built around the comparison of query gene signatures against this extensive reference collection of perturbational profiles. When a researcher submits a query signature (typically a set of up- and down-regulated genes associated with a disease state), sophisticated pattern-matching algorithms identify compounds in the database whose expression effects either mimic (positive connectivity) or reverse (negative connectivity) the query signature [46]. This capability makes CMap particularly valuable for identifying compounds that might reverse disease-associated gene expression patterns.
Computational Infrastructure and Access
To house and facilitate the use of these vast amounts of data, the Broad Institute has built CLUE (CMap and LINCS Unified Environment), a cloud-based compute infrastructure consisting of user-friendly web applications and software tools that enable researchers to access and manipulate CMap data and integrate it with their own datasets [45]. The project receives funding from the NIH LINCS (Library of Integrated Cellular Signatures) program, along with philanthropic grants, collaborative projects with industry, and Broad Institute funds [45].
Table: Evolution of Connectivity Map Resources
| Feature | CMap 1.0 | CMap 2.0 (LINCS-L1000) |
|---|---|---|
| Profiling Technology | Affymetrix GeneChips | Luminex bead arrays (L1000) |
| Directly Measured Genes | 12,010 | 978 "landmark" genes |
| Inferred Genes | None | 11,350 |
| Total Profiles | 6,100 | 591,697 |
| Compounds | 1,309 | 29,668 (including genetic perturbations) |
| Cell Lines | 5 | 98 |
CMap-Based Drug Repositioning Methodology for ASD Research
Generating ASD-Specific Query Signatures
The initial critical step in CMap-based drug repositioning for ASD involves deriving a robust gene expression signature representative of the disorder. This requires:
Identifying Differentially Expressed Genes: Using transcriptomic data from ASD studies, researchers apply statistical methods to identify genes with significant expression differences compared to neurotypical controls. For example, a study analyzing gene expression profiles from peripheral blood lymphocytes of 82 autistic patients and 64 healthy persons identified 244 genes expressed differently between the groups [14].
Network-Based Prioritization: Given ASD's genetic complexity, network analysis methods can help prioritize the most relevant genes. Research has shown that constructing gene correlation networks and analyzing structural parameters like average degree can identify genes with significant differences in network properties between autistic and healthy states [14]. These genes, which contribute most to structural differences in gene networks, are strong candidates for inclusion in the query signature.
Signature Formulation: The final signature typically consists of 50-300 of the most significantly up- and down-regulated genes, often referred to as the "query hit list." Studies suggest that signature size affects retrieval performance, with thresholds resulting in greater signature sizes (e.g., "Top-300" genes) tending to have better retrieval performance in CMap queries [47].
Query Execution and Hit Identification
Once an ASD-specific gene signature is prepared, researchers submit it to the CMap database through the CLUE platform or programmatic interfaces. The system compares this signature against all perturbation profiles in the database and returns a list of compounds ranked by connectivity scores, which quantify the similarity or reversal between the query signature and each compound's expression profile.
The most promising candidates are typically those with strongly negative connectivity scores, indicating that the compound induces expression changes opposite to the disease signature—potentially "reversing" the pathological state. For instance, in the context of ASD, a compound that upregulates genes that are underexpressed in ASD and downregulates genes that are overexpressed would represent a compelling candidate for therapeutic repurposing.
CMap Drug Repositioning Workflow for ASD Research
Advanced Computational Methods Enhancing CMap Predictions
Machine Learning and Network-Based Approaches
While traditional CMap queries rely on direct signature matching, advanced computational methods have significantly enhanced prediction accuracy and biological relevance. Machine learning approaches have become powerful tools for drug screening and target identification by enabling computational models to autonomously learn patterns and relationships from data [48]. Notable methods include:
- DTINet: Integrates data from diverse sources (drugs, proteins, diseases, side effects) and learns low-dimensional representations of drugs and proteins to manage noise, incompleteness, and high-dimensional characteristics of large-scale biological data [48].
- BridgeDPI: Incorporates "guilt-by-association" principles to enhance network-level information, effectively combining network- and learning-based approaches to improve drug-target interaction prediction [48].
- GraphIX: An explainable AI framework for drug repositioning using biological networks that learns network weights and node features using graph neural networks from known drug indications and knowledge graphs consisting of disease, drug, and protein nodes [49].
Addressing Reproducibility Challenges
A critical evaluation of CMap's performance revealed important limitations in reproducibility between different versions of the resource. Research examining the comparability of CMap 1 and CMap 2 found that when CMap 2 was queried with CMap 1-derived signatures, the success rate for prioritizing the same compound was only 17% [47]. This low reproducibility appears to be caused by limited differential expression reproducibility both between CMaps and within each CMap [47].
To mitigate these challenges, researchers should:
- Prioritize perturbations with strong differential expression signals, as DE strength was found to be predictive of reproducibility [47].
- Consider compound concentration and cell-line responsiveness, as these factors influence DE strength and reproducibility [47].
- Apply orthogonal validation using independent datasets or experimental approaches to verify computational predictions.
Table: Strategies to Enhance CMap Prediction Reliability
| Challenge | Impact on Drug Repositioning | Mitigation Strategy |
|---|---|---|
| Low DE Reproducibility | Inconsistent compound rankings between CMap versions | Focus on perturbations with strong DE signals; use consensus across multiple queries |
| Technical Variability | Reduced confidence in specific compound prioritization | Leverage orthogonal validation methods; consider biological context |
| Cell Line Context Dependency | Limited translatability to in vivo systems | Select cell lines relevant to disease pathology; use multiple cell line contexts |
| Concentration Effects | Variable expression responses at different doses | Prioritize highest concentrations available; consider dose-response relationships |
Experimental Protocols and Validation Frameworks
Core Methodological Framework for CMap Analysis
A robust protocol for CMap-based drug repositioning for ASD risk genes involves these critical methodological steps:
Signature Generation from ASD Transcriptomic Data: Begin with high-quality transcriptomic data from ASD case-control studies. Process raw data through standardized pipelines (e.g., MAS5 and RMA) for normalization. Identify differentially expressed genes using appropriate statistical methods that control for false discovery rates, incorporating network topology parameters like average degree to prioritize genes that significantly contribute to structural differences between ASD and control networks [14].
CMap Query Execution: Format the prioritized gene list into a query signature, typically consisting of 50-300 genes with the most significant up- and down-regulation. Submit this signature to the CMap/LINCS database via the CLUE web interface or programmatic API. Use multiple signature generation thresholds (e.g., top 150, top 250 genes) to assess the robustness of results.
Hit Prioritization and Triangulation: Filter results based on connectivity scores, giving priority to compounds with strongly negative scores. Apply additional filters based on compound properties (e.g., blood-brain barrier permeability, safety profile). Triangulate findings across multiple ASD datasets and signature generation methods to identify consistently ranked compounds.
Experimental Validation: Select top candidate compounds for in vitro testing using neuronal cell lines or induced pluripotent stem cell (iPSC)-derived neurons from ASD patients. Assess the ability of compounds to reverse ASD-related phenotypic endpoints, such as synaptic dysfunction, electrical activity patterns, or metabolic abnormalities.
Research Reagent Solutions for CMap Studies
Table: Essential Research Reagents and Resources for CMap-Based ASD Studies
| Reagent/Resource | Function/Application | Specifications/Considerations |
|---|---|---|
| L1000 Assay Platform | High-throughput gene expression profiling for CMap data generation | Directly measures 978 "landmark" genes; computationally infers 11,350 additional genes [45] |
| CLUE Platform | Cloud-based computational environment for CMap data access and analysis | Provides user-friendly web applications and software tools for querying and analyzing CMap data [45] |
| LINCS L1000 Dataset | Reference database of perturbational gene expression signatures | Contains >500,000 expression profiles from chemical and genetic perturbations [47] |
| ASD Transcriptomic Datasets | Source data for deriving disease-specific query signatures | Example: GSE25507 in NCBI (82 autistic patients, 64 controls) [14] |
| Graph Neural Network Frameworks | Implementation of advanced drug-target prediction algorithms | Enables network-based approaches like GraphIX for explainable drug repositioning [49] |
Future Directions and Integrative Approaches
The field of computational drug repositioning continues to evolve with several promising directions enhancing the CMap framework. Integration of multi-omics data—including genomics, epigenomics, and proteomics—provides a more comprehensive view of disease mechanisms and potential therapeutic interventions [14]. For ASD research specifically, this might involve combining CMap queries with network analyses of ASD risk genes to identify compounds that target central nodes in disrupted biological pathways.
Explainable artificial intelligence (XAI) approaches represent another significant advancement. Methods like GraphIX not only predict new disease-drug associations but also identify proteins important for understanding pharmacological effects from large and complex knowledge bases [49]. This explainability is crucial for generating biologically interpretable hypotheses and guiding subsequent validation experiments.
Emerging technologies such as large language models and AlphaFold-predicted protein structures are being integrated into drug-target interaction prediction pipelines, potentially enhancing feature engineering and improving prediction accuracy [48]. As these technologies mature, they may help address current limitations in CMap reproducibility by providing more robust biological context and structural information for interpreting compound mechanisms of action.
For ASD research, the integration of CMap with patient-specific models, such as iPSC-derived neurons and organoids, offers a path toward personalized therapeutic discovery. By deriving gene expression signatures from these patient-specific systems and querying CMap, researchers may identify compounds that reverse pathological signatures in specific genetic subtypes of ASD, moving toward more targeted and effective interventions for this heterogeneous disorder.
Addressing Heterogeneity and Optimizing Predictive Models in ASD Networks
Autism Spectrum Disorder (ASD) represents a complex array of neurodevelopmental conditions characterized by significant heterogeneity in both etiology and clinical presentation. This variability has posed a substantial challenge for researchers attempting to elucidate coherent biological mechanisms and develop targeted interventions. The prevailing understanding suggests that autism's genetic architecture encompasses hundreds of risk genes, with identified genetic causes explaining only approximately 20% of cases to date [50]. This limitation stems primarily from methodological approaches that have traditionally treated autism as a single entity rather than a collection of distinct conditions with overlapping behavioral manifestations.
Recent advances in computational analytics and large-scale data integration have enabled a paradigm shift from trait-centric to person-centered approaches. This methodological evolution allows researchers to consider the holistic phenotypic profile of individuals rather than analyzing isolated traits in isolation [3]. By leveraging broad phenotypic data matched with genomic information from large cohorts, researchers can now decompose autism's heterogeneity into biologically meaningful subtypes. This approach has revealed that phenotypic and clinical outcomes correspond to distinct genetic and molecular programs of common, de novo, and inherited variation [3] [51]. The integration of these findings within the broader context of ASD risk genes network topology research provides a powerful framework for understanding how genetic perturbations disrupt neurodevelopmental trajectories and manifest as clinically distinct subtypes.
Computational Decomposition of Phenotypic Heterogeneity
Methodological Framework and Cohort Characteristics
The identification of robust autism subtypes requires sophisticated computational approaches capable of handling multidimensional phenotypic data. Recent research has utilized a Generative Finite Mixture Model (GFMM) to analyze 239 item-level and composite phenotype features across 5,392 individuals from the SPARK cohort [3] [51]. This methodological approach minimizes statistical assumptions while accommodating heterogeneous data types (continuous, binary, and categorical) that inherently characterize autism phenotyping.
The phenotypic features incorporated in these models represent responses from standardized diagnostic instruments, including the Social Communication Questionnaire-Lifetime (SCQ), Repetitive Behavior Scale-Revised (RBS-R), Child Behavior Checklist 6-18 (CBCL), and developmental history forms focusing on milestones [3]. The GFMM framework provides an inherently person-centered approach that separates individuals into classes based on their overall phenotypic profile rather than fragmenting each individual into separate phenotypic categories [3]. Model selection involved evaluating solutions with 2-10 latent classes, with a four-class solution demonstrating optimal balance according to Bayesian Information Criterion (BIC), validation log likelihood, and clinical interpretability [3] [51].
Phenotypic Class Characteristics and Validation
The four-class solution revealed distinct phenotypic profiles that differed not only in severity of core autism symptoms but also in patterns of co-occurring cognitive, behavioral, and psychiatric features. For clinical interpretability, the 239 phenotype features were categorized into seven domains: limited social communication, restricted and/or repetitive behavior, attention deficit, disruptive behavior, anxiety and/or mood symptoms, developmental delay, and self-injury [3].
Table 1: Characteristics of the Four Autism Subtypes Identified Through Generative Mixture Modeling
| Subtype Name | Prevalence | Core Phenotypic Features | Developmental Trajectory | Co-occurring Conditions |
|---|---|---|---|---|
| Social/Behavioral Challenges | 37% (n=1,976) | High scores in social communication difficulties, restricted/repetitive behaviors, disruptive behavior, attention deficit, and anxiety | Developmental milestones similar to non-autistic children; later diagnosis | ADHD, anxiety, depression, OCD |
| Mixed ASD with Developmental Delay | 19% (n=1,002) | Nuanced presentation within core autism categories; strong enrichment of developmental delays | Later reaching of developmental milestones (walking, talking) | Language delay, intellectual disability, motor disorders |
| Moderate Challenges | 34% (n=1,860) | Consistently lower scores across all seven phenotypic categories | Developmental milestones on typical track | Generally absent co-occurring psychiatric conditions |
| Broadly Affected | 10% (n=554) | Consistently higher scores across all seven phenotypic categories | Significant developmental delays; early diagnosis | Multiple co-occurring conditions including anxiety, depression, mood dysregulation |
Validation of these phenotypic classes utilized medical history questionnaires that were not included in the original GFMM, confirming that enrichment patterns of diagnosed co-occurring conditions aligned with class-specific phenotypic profiles [3]. Furthermore, replication in an independent cohort (Simons Simplex Collection, n=861) demonstrated strong generalizability of the subtype model, with highly similar feature enrichment patterns across all seven phenotypic categories (correlation = 0.927, p < 1e-4) [3] [51].
Genetic Architecture of Autism Subtypes
Distinct Variant Profiles Across Subtypes
The phenotypic decomposition of autism enables a more precise mapping of genetic influences by reducing heterogeneity that has previously obscured genetic signals. Interrogation of the four subtypes reveals distinct patterns of common, rare inherited, and de novo variation that align with their clinical characteristics.
Table 2: Genetic Profiles Associated with Autism Subtypes
| Subtype | Common Variant Burden (PGS) | Rare Inherited Variants | De Novo Mutations | Key Biological Pathways |
|---|---|---|---|---|
| Social/Behavioral Challenges | Intermediate PGS for psychiatric conditions | Not enriched | Enriched in genes active later in childhood | Postnatal synaptic development, neuromodulatory systems |
| Mixed ASD with Developmental Delay | Not reported | Significantly enriched | Not specifically enriched | Early neurodevelopmental processes |
| Moderate Challenges | Lower PGS for related psychiatric conditions | Not enriched | Not enriched | Not specifically delineated |
| Broadly Affected | Higher PGS for neurodevelopmental conditions | Not enriched | Highest burden of damaging de novo mutations | Multiple disrupted pathways including synaptic function |
Notably, the Broadly Affected subtype demonstrated the highest proportion of damaging de novo mutations—those not inherited from either parent—while only the Mixed ASD with Developmental Delay group showed significant enrichment of rare inherited genetic variants [22]. These genetic differences suggest distinct mechanisms behind superficially similar clinical presentations, particularly for shared traits like developmental delays and intellectual disability [22].
Developmental Timing of Genetic Disruptions
Beyond specific variant types, the autism subtypes differ in the temporal patterning of when affected genes exert their effects during neurodevelopment. Genes operate within specific developmental windows, and the timing of their disruption appears to correlate with clinical trajectories across subtypes [22]. For the Social and Behavioral Challenges subtype—characterized by substantial social and psychiatric challenges without developmental delays and later diagnosis—mutations were enriched in genes that become active later in childhood [22]. This suggests that biological mechanisms may emerge postnatally for this group, aligning with their clinical presentation. Conversely, subtypes with early developmental delays (Mixed ASD with DD and Broadly Affected) demonstrated genetic disruptions in pathways active during earlier prenatal development.
Experimental Approaches for Validating Genetic Subtypes
In Vitro Models of Transcriptional Regulation
Understanding the functional consequences of genetic risk factors requires experimental models that can capture the complexity of neurodevelopmental processes while allowing for controlled manipulation of specific genes. Recent research has focused on ASD-linked transcriptional regulators, including chromatin regulators, DNA modifying enzymes, and transcription factors [33]. These nine high-confidence ASD risk genes (ASH1L, CHD8, DNMT3A, KDM6B, KMT2C, MBD5, MED13L, SETD5, and TBR1) were selected based on their SFARI gene scores and well-established associations with neurodevelopmental disorders.
The experimental protocol involves:
- Primary Neuronal Cultures: Cortical neurons derived from E16.5 embryonic mouse tissue to generate a highly pure neuron population without the complexity of brain tissue [33].
- Gene Depletion: Lentiviral delivery of shRNA at 5 days in vitro (DIV) to achieve partial depletion of targets, modeling partial loss-of-function variants.
- Transcriptional Profiling: RNA-sequencing at DIV 10 to identify differentially expressed genes (DEGs) following target depletion.
- Functional Assessment: Multielectrode array (MEA) recordings to quantify neuronal firing patterns throughout maturation.
This approach demonstrated that despite disparate functions in transcriptional regulation, disruption of these ASD-linked genes converges on shared gene expression signatures encoding critical synaptic proteins and produces consistent alterations in neuronal firing patterns [33].
Research Reagent Solutions
Table 3: Essential Research Reagents for Experimental Validation of ASD Subtypes
| Reagent/Category | Specific Examples | Research Application | Key Function |
|---|---|---|---|
| Phenotypic Assessment | SCQ, RBS-R, CBCL, SRS, SDQ, SCARED-P, ARI-P | Phenotypic decomposition | Standardized quantification of core and associated features |
| Genomic Profiling | Whole exome sequencing, Whole genome sequencing, SNP arrays, Polygenic scoring | Genetic characterization | Identification of rare and common variants associated with subtypes |
| Cell Models | Primary mouse cortical neurons (E16.5), Lentiviral shRNA delivery | Functional validation | Controlled perturbation of candidate genes in relevant cell types |
| Transcriptional Analysis | RNA-sequencing, Differential expression analysis, Gene ontology enrichment | Molecular profiling | Identification of downstream effects of genetic perturbations |
| Functional Assays | Multielectrode array (MEA) recordings, Calcium imaging, Patch clamp electrophysiology | Neuronal phenotyping | Assessment of functional consequences on neuronal activity |
Network Biology of ASD Risk Genes
Convergent Molecular Pathways
Despite hundreds of associated genes, ASD risk converges on limited biological pathways and processes. Research on nine ASD-linked transcriptional regulators revealed that despite targeting different chromatin modifications and having distinct molecular functions, their disruption leads to shared gene expression signatures in neurons [33]. These convergent signatures prominently include genes encoding synaptic proteins, suggesting that synaptic development and function represent a key hub in ASD network topology.
This convergence is particularly remarkable given the diverse functions of the tested proteins: histone methyltransferases (ASH1L, KMT2C, SETD5) targeting different histone residues, a histone demethylase (KDM6B), a chromatin remodeler (CHD8), a component of the Mediator complex (MED13L), a DNA methyltransferase (DNMT3A), a transcription factor (TBR1), and a non-catalytic chromatin-complex protein (MBD5) [33]. The finding that distinct transcriptional regulators ultimately disrupt overlapping sets of synaptic genes suggests that neurons have gene networks particularly vulnerable to transcriptional disruption.
Temporal Dynamics of Gene Networks
The network topology of ASD risk genes operates within a temporal dimension, with different genetic programs manifesting across developmental trajectories. Analysis of the four autism subtypes revealed that class-specific differences in the developmental timing of affected genes align with clinical outcome differences [3]. This temporal dimension of genetic vulnerability provides a crucial link between static genetic variation and dynamic clinical presentation.
Diagram 1: Flow of effects from genetic variants to clinical subtypes, highlighting the role of developmental timing.
Implications for Therapeutic Development
Target Identification and Stratification
The decomposition of autism into biologically distinct subtypes creates new opportunities for targeted therapeutic development. By linking specific genetic programs to clinical presentations, researchers can now pursue mechanism-based treatments tailored to particular autism subtypes. For example, the identification of the Social and Behavioral Challenges subtype as having genetic disruptions in pathways active during later childhood suggests different therapeutic windows compared to subtypes with primarily prenatal disruptions [22].
Furthermore, the convergence of diverse genetic risk factors on common synaptic pathways indicates that targeting these downstream convergent mechanisms may have efficacy across multiple genetic forms of autism [33]. This approach is particularly promising given the genetic heterogeneity of autism, where developing individualized treatments for hundreds of risk genes is impractical.
Translational Research Pipeline
The integration of phenotypic decomposition with genetic analysis creates a powerful pipeline for translational research. This pipeline begins with large-scale clinical characterization and genetic sequencing of affected individuals, proceeds through computational decomposition into subtypes, identifies subtype-specific genetic risk profiles, models these genetic effects in experimental systems, and ultimately develops and tests targeted interventions [27].
This approach represents a shift from syndrome-based to mechanism-based intervention strategies for autism. As noted by researchers, "This shift could reshape both autism research and clinical care — helping clinicians anticipate different trajectories in diagnosis, development and treatment" [22]. The ability to define biologically meaningful autism subtypes is thus foundational to realizing the vision of precision medicine for neurodevelopmental conditions.
Methodological Protocols
Phenotypic Decomposition Protocol
The identification of robust autism subtypes requires standardized methodological approaches:
- Cohort Recruitment: Large-scale (n > 5,000) cohorts with deep phenotyping and genetic data, such as SPARK or SSC [3].
- Phenotypic Assessment: Administration of standardized instruments covering core autism features (SCQ, RBS-R) and associated features (CBCL, developmental histories) [3].
- Data Processing: Item-level and composite feature extraction, resulting in 200+ phenotypic variables per individual [51].
- Generative Mixture Modeling: Application of GFMM with 2-10 latent classes, with model selection based on BIC, validation log likelihood, and clinical interpretability [3].
- Class Characterization: Assignment of phenotypic features to clinically meaningful categories and enrichment analysis to define class profiles [3].
- Validation: External validation using medical history data not included in modeling and replication in independent cohorts [3] [51].
Genetic Analysis Protocol
Following phenotypic decomposition, genetic analysis proceeds through stratified approaches:
- Polygenic Scoring: Calculation of subtype-specific polygenic scores for autism and related psychiatric conditions [3] [27].
- Rare Variant Analysis: Evaluation of de novo and inherited rare variants within and across subtypes [3] [22].
- Burden Testing: Assessment of variant burden in candidate genes and pathways for each subtype [3].
- Developmental Transcriptomics: Integration with gene expression data across developmental timelines to establish temporal patterns [3] [22].
- Pathway Analysis: Gene set enrichment analysis to identify subtype-specific biological pathways [3] [33].
Diagram 2: Workflow for deconvolving clinical heterogeneity and linking to genetic subtypes.
The decomposition of autism's clinical heterogeneity into biologically distinct subtypes represents a transformative advance in neurodevelopmental disorder research. By integrating large-scale phenotypic data with genomic information through person-centered computational approaches, researchers have identified four robust subtypes with distinct clinical trajectories and genetic architectures. These subtypes demonstrate specific patterns of common, rare inherited, and de novo variation that align with their phenotypic profiles, and reveal subtype-specific disruptions in biological pathways and developmental timing.
This refined nosology enables more precise mapping of ASD risk genes within network topologies, revealing convergent molecular pathways despite genetic heterogeneity. The experimental validation of these subtypes using primary neuronal models demonstrates functional convergence across distinct genetic perturbations, particularly in synaptic pathways. These advances create new opportunities for targeted therapeutic development and establish a framework for precision medicine in autism that moves beyond behavioral symptomatology to address underlying biological mechanisms.
The continued refinement of autism subtypes through increased sample sizes, improved ancestral diversity, and deeper phenotyping will further enhance our understanding of the complex relationship between genetic variation, neurodevelopment, and clinical presentation. This approach provides a template for addressing heterogeneity in other complex neuropsychiatric conditions and represents a crucial step toward biologically-informed diagnosis and treatment for individuals with autism.
The integration of machine learning with network science is revolutionizing the study of complex neurodevelopmental disorders. This technical guide details the application of Random Forest classifiers to network features derived from Autism Spectrum Disorder (ASD) risk gene research. We present validated methodologies demonstrating how network topology data can enhance predictive models for identifying key genetic contributors and functional consequences. Our protocols enable researchers to leverage ensemble learning for robust, interpretable analysis of biological networks, accelerating the translation of genomic findings into therapeutic insights.
Random Forest is an ensemble machine learning method that constructs multiple decision trees during training and outputs the mode of classes (classification) or mean prediction (regression) of the individual trees [52]. This algorithm operates by creating numerous decision trees, each using random subsets of both data instances and features, with final predictions determined through majority voting for classification tasks [52]. The inherent properties of Random Forest make it particularly suitable for analyzing network features in biological contexts, especially for ASD risk gene research where data complexity and multidimensional interactions present significant analytical challenges.
In the context of network topology research, Random Forest classifiers provide distinct advantages for identifying meaningful patterns within interconnected biological systems. The algorithm's capacity to handle high-dimensional data without requiring feature scaling accommodates the complex feature spaces typical of network topological measures [52]. Furthermore, its ability to quantify feature importance offers crucial interpretability, allowing researchers to identify which network properties most significantly contribute to ASD risk classification [52]. This capability aligns perfectly with the need to pinpoint critical nodes and connections within biological networks associated with neurodevelopmental outcomes.
Theoretical Foundations: ASD Risk Genes and Network Topology
ASD exemplifies a complex disorder with numerous genetic risk factors, many encoding transcriptional regulators that converge on common functional pathways [33]. Recent evidence indicates that disparate ASD-linked genes frequently disrupt coherent biological networks, particularly those involving synaptic function and neuronal communication. Independent studies have identified shared gene expression signatures across multiple ASD risk genes that converge on disruption of critical synaptic genes, with corresponding effects on neuronal firing patterns throughout maturation [33].
Network topology analysis provides a powerful framework for understanding how genetic perturbations propagate through biological systems to produce phenotypic outcomes. Research has demonstrated that intrinsic functional connectivity of large-scale brain networks differs significantly in children with ASD, with particular promise shown by salience network connectivity for discriminating ASD from typically developing children with 78% classification accuracy [53]. These network-level alterations represent accessible intermediate phenotypes between genetic risk and behavioral manifestations.
The integration of network features into machine learning models creates opportunities to identify multivariate patterns that individual biomarkers cannot capture. For instance, hyperconnectivity patterns encompassing salience, default mode, frontotemporal, motor, and visual networks have been replicated across independent cohorts of children with ASD [53]. Quantifying these network properties generates feature spaces ideally suited for Random Forest classification, enabling researchers to distinguish pathological network configurations from typical developmental patterns.
Implementation Protocol: Random Forest for Network Feature Classification
Data Preparation and Feature Engineering
The foundation of effective Random Forest classification lies in meticulous data preparation. For ASD risk gene network research, this process involves multiple critical stages:
Network Feature Extraction: Calculate topological measures from biological networks, including betweenness centrality, degree distribution, clustering coefficients, and connection density. For fMRI-based functional connectivity networks, extract correlation strengths between predefined regions of interest [53] [54]. For genetic interaction networks, quantify protein-protein interaction scores and co-expression patterns.
Feature Selection: Employ a multi-strategy approach to identify optimal feature subsets. Combine correlation analysis (excluding features with |r| < 0.1), chi-square tests (p < 0.05), LASSO regression, and Random Forest-based importance ranking [55]. This hybrid methodology captures both linear and non-linear relationships, enhancing model generalizability across diverse datasets.
Data Preprocessing: Address missing values through appropriate imputation strategies—mean imputation for continuous variables (e.g., connectivity strength) and mode imputation for categorical variables. Standardize numerical features using Z-score normalization (mean = 0, standard deviation = 1) to ensure uniform feature scaling despite different measurement units [55]. Encode categorical variables using one-hot encoding to avoid imposing ordinal relationships where none exist.
Random Forest Classifier Configuration
Implement the Random Forest classifier with parameters optimized for network topology data:
Model Evaluation and Interpretation
Comprehensive model assessment requires multiple validation strategies:
Performance Metrics: Evaluate using accuracy, precision, recall, F1-score, and area under the ROC curve (AUC-ROC). For ASD classification studies, prioritize sensitivity to ensure identification of true positive cases while maintaining specificity [54].
Feature Importance Analysis: Extract and visualize feature importance scores to identify which network topology measures contribute most significantly to classification. This provides biological insights beyond predictive accuracy alone [52].
Cross-Validation: Implement k-fold cross-validation (typically k=5 or k=10) to assess model stability and mitigate overfitting, particularly crucial with limited sample sizes common in neurobiological studies.
Experimental Results and Comparative Analysis
Performance Benchmarking
Application of Random Forest classifiers to ASD network data demonstrates compelling performance across multiple studies:
Table 1: Comparative Performance of Random Forest in ASD Classification Studies
| Study | Data Modality | Sample Size | Key Features | Accuracy | AUC-ROC |
|---|---|---|---|---|---|
| DNN + DDPG [55] | Behavioral & Demographic | Multi-cohort | Qchat-10-Score, Ethnicity | 96.98% | 99.75% |
| MADE-for-ASD [54] | rs-fMRI (ABIDE I) | 17 sites | Multi-atlas integration | 75.20% | - |
| Salience Network [53] | fMRI Connectivity | 40 children | Salience network connectivity | 78.00% | - |
| Conventional RF [52] | Structured clinical | Titanic dataset | Class, Sex, Age | ~80% (example) | - |
Key Network Topology Predictors
Research consistently identifies specific network features as significant predictors in ASD classification:
Table 2: High-Value Network Features for ASD Classification
| Feature Category | Specific Measures | Biological Interpretation | Study Reference |
|---|---|---|---|
| Functional Connectivity | Salience network hyperconnectivity | Attention allocation to socially relevant stimuli | [53] |
| Default mode network connectivity | Self-referential thought | [53] | |
| Frontotemporal connectivity | Social communication processing | [53] | |
| Gene Co-expression | Synaptic gene expression signature | Neuronal communication integrity | [33] |
| Transcriptional regulator targets | Chromatin remodeling effects | [33] | |
| Demographic & Behavioral | Qchat-10-Score | Early ASD trait quantification | [55] |
| Social Responsiveness Scale | Social behavior impairment | [55] |
Research Reagent Solutions
Table 3: Essential Research Reagents and Computational Tools
| Reagent/Tool | Function | Application Context |
|---|---|---|
| shRNA Lentivirus | Partial depletion of transcriptional regulators | Modeling loss-of-function variants in neuronal cultures [33] |
| Primary Neuronal Cultures | Genetically identical neuron populations | Controlled testing of ASD gene effects [33] |
| RNA-sequencing | Transcriptome profiling | Identifying differentially expressed genes [33] |
| Multielectrode Array (MEA) | Neuronal firing pattern recording | Functional assessment of network activity [33] |
| scikit-learn Library | Random Forest implementation | Accessible machine learning framework [52] |
| ABIDE Dataset | Multi-site fMRI data | Standardized neuroimaging benchmark [54] |
| PARTNER CPRM | Network mapping and analysis | Network visualization and topological analysis [56] |
Workflow Visualization
Random Forest classifiers applied to network topology features represent a powerful methodology for advancing ASD risk gene research. The ensemble approach effectively handles the high-dimensional, interconnected nature of biological network data while providing interpretable feature importance metrics. Integration of multimodal data—from genetic interactions and functional brain connectivity to behavioral measures—within this framework enables robust identification of multivariate patterns associated with ASD pathogenesis. The protocols and analyses presented in this guide provide researchers with practical tools for implementing these approaches, potentially accelerating the discovery of novel therapeutic targets and biomarkers for early intervention.
Autism spectrum disorder (ASD) represents a profoundly heterogeneous neurodevelopmental condition with a complex genetic architecture. While substantial evidence confirms a strong genetic basis for ASD—with heritability estimates ranging from 64% to 91%—current genetic understanding remains disproportionately shaped by studies of European ancestry populations [57] [58]. This ancestry bias creates critical gaps in both biological knowledge and clinical translation, limiting the generalizability of findings and perpetuating health disparities. Cross-ancestry genetic analysis emerges as an essential methodological framework to overcome these limitations, expanding the genetic landscape of ASD by capturing a more comprehensive spectrum of risk variants across diverse populations. Such approaches are particularly vital for ASD, where hundreds of risk genes have been identified yet collectively explain only a fraction of cases, and where significant phenotypic heterogeneity suggests the existence of ancestry-specific modifying factors [27] [3].
The technical rationale for cross-ancestry approaches extends beyond mere inclusion. Differences in linkage disequilibrium patterns across ancestral groups can enhance fine-mapping resolution, while varying allele frequencies can reveal novel associations obscured in homogeneous cohorts. Furthermore, evidence suggests that genetic liability for ASD manifests differently across sexes and ancestries, with current diagnostic instruments potentially reflecting male-biased criteria that underestimate prevalence in females and possibly in diverse populations [59] [60]. This whitepaper synthesizes current methodologies, findings, and experimental frameworks in cross-ancestry ASD genetics, providing researchers with advanced tools to expand and refine our understanding of ASD's genetic architecture across human diversity.
Current Landscape and Challenges in ASD Genetics
The Complexity of ASD Genetic Architecture
ASD's genetic architecture encompasses an intricate spectrum of risk variants, including rare de novo mutations, copy number variations (CNVs), and common variants with small effect sizes. Large-scale sequencing studies have identified numerous highly reliable risk loci, though no single mutation accounts for more than 1% of ASD cases, reflecting exceptional heterogeneity [57]. Notably, a disproportionate number of ASD risk genes encode transcriptional regulators, including chromatin modifiers, transcription factors, and DNA methylation machinery, which converge on disrupting critical synaptic genes and neuronal firing patterns [33]. This functional convergence suggests that diverse genetic perturbations may affect common biological pathways, yet mapping these networks requires sufficiently diverse datasets to capture the full spectrum of relevant variation.
Ancestry Bias in Existing Genetic Studies
Current ASD genetic resources suffer from significant ancestry imbalance. Most large-scale genome-wide association studies (GWAS) and whole-exome sequencing initiatives have predominantly featured participants of European descent, creating a genetic reference landscape that fails to represent global diversity. The consequences of this bias extend beyond equity concerns to fundamentally limit biological understanding. Studies in other complex traits have demonstrated that ancestry-specific variants can reveal novel biological pathways, yet similar systematic approaches in ASD remain nascent [61] [62]. Furthermore, the assessment instruments and diagnostic criteria themselves may reflect cultural and sex biases that affect ascertainment across populations, potentially obscuring the true genetic architecture of ASD [59].
Table 1: Key Challenges in ASD Cross-Ancestry Genetic Analysis
| Challenge | Impact on Genetic Discovery | Potential Solutions |
|---|---|---|
| Ancestral Imbalance in Samples | Limited portability of polygenic risk scores; missed ancestry-specific risk variants | Purposeful recruitment of diverse cohorts; consortium-based data sharing |
| Phenotypic Heterogeneity | Inconsistent genotype-phenotype relationships across populations | Deep phenotyping; development of culturally appropriate assessment tools |
| Methodological Limitations | Inadequate statistical power for variant detection in non-European groups | Advanced Bayesian methods; cross-ancestry meta-analysis frameworks |
| Complex Gene-Environment Interactions | Differential expression of genetic risk across populations | Integrated multi-omics approaches; careful covariate adjustment |
Methodological Frameworks for Cross-Ancestry Analysis
Genomic Data Integration and Quality Control
Robust cross-ancestry analysis begins with rigorous data harmonization across diverse cohorts. Essential preprocessing steps include genomic coordinate unification (e.g., using CrossMap v0.6.5 with UCSC chain files for hg19-to-hg38 conversion), careful allele alignment against reference panels (e.g., 1000 Genomes Phase 3 using PLINK v1.9), and population structure assessment via principal component analysis [57]. For meta-analysis across multiple studies, the fixed-effects model in METAL implements SCHEME STDERR and STDERR SE strategies to weight data appropriately, with AVERAGEFREQ and MINMAXFREQ options enabled to exclude SNPs with cross-study effective allele frequency differences >0.2, ensuring allele frequency consistency across diverse samples [57]. Heterogeneity assessment through Cochran's Q and I² indices is critical, with random-effects models (e.g., DerSimonian-Laird method) applied when significant heterogeneity is detected (Q test P < 0.1 and I² > 50%) [57].
Novel Locus Discovery and Validation
In cross-ancestry frameworks, novel locus identification requires specialized approaches to distinguish genuinely novel associations from ancestry-specific signals. Established protocols define novel loci as SNPs located ≥500 kilobases from previously reported loci on the same chromosome, with linkage disequilibrium pruning (retaining independent SNPs with r² < 0.001 within 10,000-kilobase windows) [57]. Functional validation incorporates Polygenic Priority Score (PoPS) analysis, tissue-specific expression quantitative trait loci (eQTL) enrichment, and Summary-data-based Mendelian Randomization (SMR) integrating brain cis-eQTL and methylation QTL data to prioritize variants with multidimensional support [57]. For genes, annotation using resources like biomaRt connected to Ensembl (GRCh38) captures genes within 500kb upstream and downstream of each novel locus [57].
Advanced Network and Machine Learning Approaches
Graph-based computational methods have emerged as powerful tools for identifying ASD risk genes across diverse populations. Protein-protein interaction networks can be constructed with genes as nodes, chromosome band locations as node features, and gene interactions as edges [58]. Graph neural network architectures—including Graph Sage, graph convolutional networks, and graph transformers—can then classify ASD risk association through three primary tasks: binary risk association (gene with/without associated risk), multi-class risk association (no, low, moderate, or high gene association), and syndromic gene classification (syndromic vs. non-syndromic) [58]. These approaches leverage the fundamental biological insight that ASD risk genes tend to be functionally related and converge on molecular networks and biological pathways implicated in disease, even across ancestral groups [58].
Table 2: Analytical Tools for Cross-Ancestry ASD Genetics
| Tool Category | Specific Software/Approach | Application in Cross-Ancestry Context |
|---|---|---|
| Variant Association | REGENIE v3.3 [61] | Gene-burden association tests across ancestries with optimal calibration |
| Meta-Analysis | METAL [57] [62] | Fixed-effects and random-effects models for cross-study integration |
| Functional Mapping | FUMA [62] | Functional mapping and gene-based analysis for diverse cohorts |
| Network Analysis | Graph Neural Networks [58] | Protein interaction network-based risk prediction |
| Genetic Correlation | LDSC, Popcorn [62] | Estimating genetic correlations across populations |
Key Experimental Protocols for Cross-Ancestry ASD Research
Cross-Ancestry Meta-Analysis of GWAS Data
Objective: Identify novel genetic loci associated with ASD across diverse ancestral backgrounds.
Sample Collection and Preparation:
- Obtain GWAS data from multiple independent ASD cohorts with diverse ancestry representation
- Ensure each dataset includes adequate sample size (cases and controls) with ancestry metadata
- Apply uniform quality control: SNP call rate >98%, sample call rate >95%, Hardy-Weinberg equilibrium P > 1×10⁻⁶, minor allele frequency >1%
Genomic Coordination and Alignment:
- Utilize CrossMap (v0.6.5) with UCSC chain files (hg19ToHg38.chain) to convert genomic coordinates to build GRCh38
- Employ PLINK (v1.9) to align alleles to the 1000 Genomes Phase 3 reference panel
- Correct allele direction and exclude mismatched SNPs
Meta-Analysis Execution:
- Implement fixed-effects model in METAL using SCHEME STDERR and STDERR SE strategies
- Enable AVERAGEFREQ and MINMAXFREQ options to exclude SNPs with cross-study eAF differences >0.2
- Calculate Cochran's Q and I² indices to assess heterogeneity
- Apply random-effects model (DerSimonian-Laird method) when significant heterogeneity is detected (Q test P < 0.1 and I² > 50%)
Novel Locus Identification:
- Merge meta-analysis results with pre-processed data from original sources
- Exclude known loci (SNPs within ±500 kb of previously reported loci)
- Perform linkage disequilibrium pruning (r² < 0.001 within 10,000-kb window)
- Select variants with P < 5×10⁻⁶ for further validation [57]
Multi-Omics Integration for Cross-Tissue Regulation
Objective: Elucidate cross-tissue regulatory mechanisms through integrated analysis of genomic, transcriptomic, and epigenomic data.
Data Acquisition:
- Obtain brain cis-eQTL and methylation QTL data from relevant repositories (e.g., GTEx, BRAINEAC)
- Acquire blood eQTL data to identify immune pathway associations
- Collect gut microbiota GWAS data (abundance of 473 microbial taxonomic groups)
Analytical Integration:
- Conduct Summary-data-based Mendelian Randomization (SMR) to test pleiotropic associations
- Perform bidirectional Mendelian Randomization between gut microbiota composition and ASD
- Integrate Polygenic Priority Score (PoPS) analysis with tissue-specific eQTL enrichment
- Identify SNPs with significant multi-dimensional associations across omics layers [57]
Phenotypic Decomposition and Genetic Correlation
Objective: Deconstruct phenotypic heterogeneity into biologically meaningful classes with distinct genetic architectures.
Phenotypic Data Collection:
- Collect item-level and composite phenotype features from standardized instruments (SCQ, RBS-R, CBCL)
- Include developmental history, cognitive assessments, and co-occurring condition diagnoses
- Ensure sufficient sample size (n > 5,000 recommended) for robust class discovery
Mixture Modeling:
- Implement General Finite Mixture Model (GFMM) to accommodate heterogeneous data types (continuous, binary, categorical)
- Evaluate model fit using Bayesian Information Criterion (BIC), validation log likelihood, and clinical interpretability
- Select optimal number of classes (typically 3-5) balancing statistical and clinical considerations
Genetic Correlation Analysis:
- Calculate polygenic risk scores for each phenotypic class
- Assess enrichment of de novo and rare inherited variation across classes
- Identify class-specific gene expression patterns during development [3]
Figure 1: Integrated Workflow for Cross-Ancestry ASD Genetic Analysis. This framework combines genomic and phenotypic approaches to elucidate the genetic architecture of ASD across diverse populations.
Research Reagent Solutions for Cross-Ancestry Studies
Table 3: Essential Research Reagents and Resources for Cross-Ancestry ASD Genetics
| Reagent Category | Specific Resources | Application in Cross-Ancestry Research |
|---|---|---|
| Genomic Reference | 1000 Genomes Phase 3 [57] | Ancestry-aware variant alignment and imputation |
| ASD Gene Databases | SFARI Gene Module [58] [33] | Reference for high-confidence ASD risk genes |
| Protein Interaction | Protein Interaction Network (PIN) [58] | Construction of gene networks for risk prediction |
| Expression Atlas | GTEx, BrainSpan [61] [27] | Tissue-specific expression quantitative trait loci |
| Phenotypic Instruments | SCQ, RBS-R, CBCL, ADOS [3] | Standardized phenotypic assessment across populations |
| Computational Tools | METAL, REGENIE, FUMA, Graph Neural Networks [57] [58] [61] | Statistical analysis and network-based discovery |
Signaling Pathways and Biological Convergence
Despite substantial genetic heterogeneity, cross-ancestry analyses reveal remarkable convergence of ASD risk genes onto specific biological pathways and processes. Multiple lines of evidence indicate that distinct genetic perturbations disrupt common neurodevelopmental programs, particularly those governing synaptic function, chromatin remodeling, and neuronal connectivity [33]. Functional studies of nine ASD-linked transcriptional regulators (ASH1L, CHD8, DNMT3A, KDM6B, KMT2C, MBD5, MED13L, SETD5, and TBR1) demonstrated that despite targeting different molecular processes—including histone modification, chromatin remodeling, and transcription factor activity—their depletion in neuronal cultures produced shared gene expression signatures affecting critical synaptic genes [33]. This convergence suggests that therapeutic interventions targeting these final common pathways may benefit genetically diverse individuals with ASD.
The "gut microbiota-immune-brain axis" represents another crucial pathway emerging from integrated omics studies. Specific genetic loci (e.g., rs2735307 and rs989134) participate in gut microbiota regulation while simultaneously involving immune pathways such as T cell receptor signaling and neutrophil extracellular trap formation [57]. These same loci appear to cis-regulate neurodevelopmental genes (HMGN1 and H3C9P) and influence epigenetic methylation modifications regulating BRWD1 and ABT1 expression, establishing a compelling cross-tissue regulatory network [57]. This multi-system perspective underscores how genetic variants can coordinate dynamic balance between brain development, immune responses, and gut microbiome interactions—offering novel targets for intervention that acknowledge the systemic nature of ASD.
Figure 2: Convergent Biological Pathways in ASD Genetics. Cross-ancestry analyses reveal how diverse genetic variants disrupt common neurodevelopmental processes through interconnected systems.
Cross-ancestry genetic analysis represents both an ethical imperative and a scientific opportunity to transform our understanding of ASD's complex genetic architecture. By moving beyond predominantly European cohorts to embrace global genetic diversity, researchers can discover novel risk variants, improve fine-mapping resolution, and develop more inclusive diagnostic and therapeutic approaches. The methodological frameworks outlined in this whitepaper—including advanced meta-analysis techniques, multi-omics integration, phenotypic decomposition, and network-based approaches—provide researchers with powerful tools to overcome historical biases in ASD genetics.
Future progress will require concerted effort across multiple domains: expanded recruitment of diverse populations into genetic studies, development of culturally appropriate assessment tools, refinement of statistical methods for cross-ancestry analysis, and increased collaboration across international research consortia. Particularly promising directions include integrating cross-ancestry findings with single-cell omics technologies to resolve cellular-specific effects, applying advanced machine learning methods to model gene-environment interactions across diverse populations, and developing ancestry-aware therapeutic development pipelines. As these approaches mature, they will illuminate the full spectrum of ASD genetic architecture and ensure that precision medicine benefits extend to all individuals with autism, regardless of ancestry.
Within the broader thesis on Autism Spectrum Disorder (ASD) risk genes network topology research, the identification of reliable genetic predictors remains a fundamental challenge. ASD is a complex neurodevelopmental disorder with a strong genetic basis, thought to be influenced by hundreds to over a thousand genes [19] [63]. While traditional gene-level predictors have provided valuable insights, emerging network-based methods that incorporate biological context offer promising alternatives. This technical guide provides a comprehensive benchmarking analysis of these approaches, evaluating their performance in prioritizing ASD risk genes based on quantitative metrics, methodological rigor, and practical applicability for researchers and drug development professionals. The integration of network topology within this evaluation framework allows for assessing how biological context improves gene discovery.
Performance Benchmarking: Quantitative Comparison of Prediction Methods
The table below summarizes the performance metrics of various ASD risk gene prediction methods as reported in validation studies.
Table 1: Performance comparison of ASD risk gene prediction methods
| Method Name | Core Methodology | Validation Dataset | Key Performance Metrics | Comparative Advantage |
|---|---|---|---|---|
| Network Propagation with Random Forest [64] | Network propagation on PPI network integrated with random forest | SFARI genes (Category 1 as positives) | AUROC: 0.87, AUPRC: 0.89 | Outperformed forecASD (AUROC: 0.87 vs. 0.82) |
| forecASD [63] | Stacked random forest ensemble combining BrainSpan expression and STRING networks | MSSNG, ASC, and SPARK sequencing studies | Superior performance in prioritizing de novo mutations in three independent tests | Effectively integrates multiple data types and previous association scores |
| ASD-Risk [65] | SVM with feature selection on brain temporospatial expression | BrainSpan atlas (13 developmental stages, 26 brain structures) | 10-CV Accuracy: 81.83%, Sensitivity: 0.84, Specificity: 0.79, AUC: 0.84 | Identifies critical brain developmental windows for ASD risk |
| Brain-Specific FRN [66] | Random forest on Bayesian-integrated cross-species functional relationship network | 5,000+ simplex and multiplex ASD families | Effective prioritization of de novo mutations in large validation cohorts | Incorporates multi-species brain-specific molecular data |
| Stacking-SMOTE [67] | Hybrid stacking ensemble with synthetic minority oversampling | SFARI gene database with GO annotations | Accuracy: 95.5%, addresses class imbalance | Effectively handles dataset imbalance common in ASD genetics |
Table 2: Data types and biological features utilized by different prediction approaches
| Method Category | Genetic Variation Features | Expression Data | Network Data | Other Biological Context |
|---|---|---|---|---|
| Network-Based Methods | Gene-level constraint metrics (pLI, Z-scores) [19] | BrainSpan spatiotemporal data [19] [63] | Protein-protein interactions [64] [66] | Gene Ontology annotations [67] |
| Traditional Gene-Level Predictors | De novo mutation burden [19] | General gene expression | Limited or no network context | Functional enrichment |
Methodological Protocols for Network-Based Gene Prediction
Network Propagation with Random Forest Integration
This protocol involves a two-stage process for associating genes with ASD through network propagation and machine learning classification [64].
Input Preparation:
- Compile ASD-associated gene lists from genomic, transcriptomic, proteomic, and phosphoproteomic studies
- Obtain human protein-protein interaction network data (e.g., 20,933 proteins and 251,078 interactions)
Network Propagation:
- Initialize seed proteins from ASD gene lists with values set to 1/s (where s is list size)
- Perform network propagation with damping parameter α = 0.8
- Normalize results using eigenvector centrality to correct for node degree bias
- Generate ten propagation scores for each gene to comprise initial feature set
Random Forest Classification:
- Train model using SFARI Gene Scoring categories (Category 1 as positives, randomly selected non-ASD genes as negatives)
- Implement random forest with 100 trees, no maximum depth, and minimum samples split of 2
- Validate through 5-fold cross-validation
- Calculate optimal classification cutoff (e.g., 0.86) maximizing specificity and sensitivity product
forecASD Stacked Ensemble Method
The forecASD methodology employs a two-level stacked ensemble approach to integrate diverse genomic data types [63].
Level 1 - Feature Generation:
- BrainSpan Processing: Filter brain regions with <20 samples, smooth expression across timepoints, linearly interpolate to 50 standardized timepoints, z-scale expression values to generate 800 features per gene (16 regions × 50 timepoints)
- STRING Network Processing: Convert interaction scores to shortest paths matrix, remove low-confidence interactions (score <0.4), calculate shortest paths between all gene pairs
Level 1 - Model Training:
- Train separate random forest models on BrainSpan features and STRING shortest paths
- Use SFARI high-confidence genes (n=76) as positives and 1,000 random non-SFARI genes as negatives
- Implement balanced sampling (70 positive and 70 negative examples per tree)
- Perform backward elimination feature selection for STRING model
Level 2 - Ensemble Integration:
- Extract predictions from Level 1 models as features
- Incorporate additional features: TADA scores from ASD meta-analyses
- Train final random forest model on combined feature set
- Generate genome-wide forecASD scores indexing ASD association evidence
Cross-Species Functional Relationship Network Construction
This protocol details the creation of a brain-specific functional relationship network for ASD gene prediction [66].
Data Collection:
- Microarray data: 213 non-cancer brain tissue datasets from human, mouse, and rat
- Protein-protein interactions: Aggregate from BIND, BioGRID, IntAct, MINT, MIPS
- Protein docking: Calculate quantitative physical interaction scores for mouse isoforms, map to human homologs
- Phenotype annotations: Obtain from Mouse Genome Informatics database
Bayesian Integration:
- Implement two-layer Bayesian network for weighted data integration
- Calculate posterior probability of functional interaction using formula: P(FR|E₁,E₂,E₃,...,Eₙ) = (1/C)P(FR)Πᵢ₌₁ⁿ P(Eᵢ|FR)
- Assign dataset weights based on reliability against gold standard functional relationships
Random Forest Implementation:
- Use high-confidence ASD truth set (143 genes from SFARI and Sanders list)
- Train on functional connections between known ASD genes
- Generate genome-wide ranking of ASD candidate genes
Visualizing Methodologies and Biological Workflows
Network Propagation and Random Forest Classification
Network Propagation RF Workflow: This diagram illustrates the complete workflow for network propagation with random forest classification, from data preparation to final gene rankings.
forecASD Stacked Ensemble Architecture
forecASD Ensemble Architecture: This visualization shows the two-level stacked ensemble architecture of the forecASD method, which integrates multiple data types and model predictions.
Table 3: Essential research reagents and databases for ASD risk gene prediction
| Resource Name | Type | Primary Function | Application in ASD Research |
|---|---|---|---|
| SFARI Gene Database [64] [66] [67] | Manually curated database | Provides expert-curated ASD gene classifications | Gold standard for training and validating prediction models |
| BrainSpan Atlas [65] [19] [63] | RNA-Seq database | Developmental transcriptome of human brain | Provides spatiotemporal expression features for prediction |
| STRING Database [26] [64] [63] | Protein-protein interaction network | Documents functional associations between proteins | Network construction and feature generation |
| Gene Ontology (GO) [67] | Functional annotation database | Standardized gene function representations | Semantic similarity calculations between genes |
| ExAC/gnomAD [19] | Population genomic database | Gene-level constraint metrics (pLI, Z-scores) | Features indicating gene intolerance to variation |
Discussion and Research Implications
The benchmarking analysis reveals that network-based methods consistently outperform traditional gene-level predictors in prioritizing ASD risk genes. The superior performance of these approaches can be attributed to their ability to integrate multiple data types within biologically relevant contexts, particularly brain-specific networks and developmental trajectories. Network propagation with random forest classification achieves an AUROC of 0.87, significantly exceeding previous methods [64], while the forecASD ensemble approach demonstrates robust performance across multiple independent validation cohorts [63].
The practical implications for drug development are substantial. Network-based methods identify not only individual risk genes but also entire biological pathways and modules that represent promising therapeutic targets. For instance, functional enrichment analyses of genes prioritized by these methods consistently highlight pathways involved in chromatin remodeling, synaptic function, and ubiquitination [19] [68]. Furthermore, the identification of critical spatiotemporal windows in brain development provides valuable guidance for timing therapeutic interventions.
Future methodological developments should focus on incorporating additional data types, including single-cell expression profiles, chromatin interaction data, and clinical phenotypic information. Additionally, methods that explicitly model the dynamic nature of biological networks across development may further enhance prediction accuracy and biological relevance. The integration of network-based ASD gene predictions with functional genomic data from human genetics studies represents a promising path toward personalized therapeutic strategies.
Validating Network Findings and Translating Insights into Clinical Applications
Within autism spectrum disorder (ASD) research, the identification of risk genes has increasingly relied on network-based approaches that map the complex protein-protein interaction (PPI) landscape. These analyses often yield large-scale gene modules whose biological significance requires rigorous validation. Functional Enrichment Analysis (FEA) serves as the critical computational biology method for interpreting these findings by identifying biological pathways and functions that are overrepresented in a gene module more than would be expected by chance [69]. In the context of ASD—a condition with extreme genetic heterogeneity and an estimated 500+ contributing genes—FEA provides a systematic framework to distill complex gene lists into coherent biological narratives, ultimately illuminating shared pathological mechanisms across seemingly disparate genetic mutations [70] [66]. This technical guide outlines comprehensive methodologies for applying FEA specifically to validate gene modules derived from ASD network topology research, providing experimental protocols, analytical frameworks, and interpretation guidelines tailored to researchers, scientists, and drug development professionals in the field.
Core Principles of Functional Enrichment Analysis
Analytical Approaches in FEA
Functional enrichment methodologies can be broadly categorized into distinct analytical approaches, each with specific applications and underlying statistical assumptions:
Table 1: Comparison of Functional Enrichment Analysis Methods
| Method Type | Key Features | Input Data Format | Null Hypothesis | Example Tools |
|---|---|---|---|---|
| Overrepresentation Analysis (ORA) | Uses a strict cutoff to create gene lists from experimental data; identifies pathways enriched in these lists | Non-ranked gene list | Self-contained: Genes in the list are equally associated with the phenotype | g:Profiler [69], Enrichr [69] |
| Gene Set Enrichment Analysis (GSEA) | Considers the entire ranked gene list; identifies pathways enriched at the extremes of the ranking | Ranked gene list | Competitive: Genes in the pathway are more associated with the phenotype than genes not in the pathway | GSEA [71] [69], fGSEA [72] |
| Topology-Based PEA (TPEA) | Incorporates network structure and interactions between genes | Gene list with interaction data | Accounts for pathway topology | CePa [69], SPIA [69] |
ORA methods are particularly valuable when researchers have clear, pre-defined gene sets from network module detection algorithms. For instance, when a protein interaction network analysis identifies a tightly interconnected module of 50 genes from ASD exome sequencing data, ORA can determine which biological functions are statistically overrepresented in this specific group compared to the background genome [70]. In contrast, GSEA does not require arbitrary significance cutoffs and instead works with genome-wide ranked data (e.g., genes ranked by expression fold change or mutation significance), making it ideal for detecting subtle but coordinated expression changes across pathway members [71] [72]. Topology-based methods represent a more advanced approach that incorporates the physical and functional relationships between genes within pathways, though these methods require high-quality interaction data and can be sensitive to incomplete network annotations [69].
Selection of Background and Annotation Databases
The choice of appropriate background sets and annotation databases fundamentally influences FEA results. The background set (or reference set) represents the universe of possible genes that could have been selected in the experiment, which for ASD network studies typically consists of all genes expressed in relevant neurological contexts rather than the whole genome [66]. Annotation databases provide the pathway definitions against which query gene sets are tested:
- Gene Ontology (GO): Provides structured, hierarchical annotations across biological processes, molecular functions, and cellular components [69] [73].
- KEGG (Kyoto Encyclopedia of Genes and Genomes): Curated pathway maps representing molecular interaction networks [70] [66].
- Reactome: Manually curated peer-reviewed pathway database with detailed molecular transitions [72] [69].
- MSigDB (Molecular Signatures Database): Large collection of annotated gene sets for use with GSEA [71].
- Brain-specific annotations: Custom gene sets derived from brain expression data are particularly valuable for ASD applications [66].
FEA Application in ASD Network Research: Experimental Protocols
Protocol 1: Network Propagation with Random Forest Integration for ASD Gene Prediction
Objective: To prioritize novel ASD risk genes by integrating multiple genomic datasets through network propagation and machine learning.
Methodology Details: This approach constructs a computational pipeline with two primary stages: network-based feature generation followed by random forest classification [30].
Step 1: Feature Generation via Network Propagation
- Collect ten ASD-related gene sets from various genomic studies (e.g., differential expression, alternative splicing, copy number variation) comprising approximately 4,615 unique genes [30].
- Use each gene list as a seed for network propagation within a human PPI network containing 20,933 proteins and 251,078 interactions.
- Apply a damping parameter (α=0.8) during propagation to control the influence distance from seed genes.
- Normalize results using eigenvector centrality to correct for node degree bias.
- Generate ten propagation scores per gene, creating a comprehensive feature set capturing network proximity to different ASD genomic signatures.
Step 2: Random Forest Classification
- Utilize SFARI Gene Scoring categories as truth labels: "Category 1" genes as positives (n=206) and randomly select an equal number of negatives from genes not in SFARI database.
- Train a random forest model using Python's "sklearn" package with default parameters (100 maximum trees, no maximum depth).
- Validate through 5-fold cross-validation, achieving mean AUROC of 0.87 and AUPRC of 0.89.
- Establish an optimal classification cutoff of 0.86 maximizing specificity and sensitivity product.
Interpretation: The high performance metrics demonstrate that network-propagation derived features effectively capture biologically meaningful signals for ASD gene prediction. The model successfully prioritized SFARI categories 2 and 3 genes not used in training (p-value < 3.62e-34), validating its predictive capability for novel ASD associations [30].
Network Propagation and Random Forest Workflow for ASD Gene Prediction
Protocol 2: Neuron-Specific Protein Interaction Mapping for ASD Risk Genes
Objective: To identify cell type-specific protein interaction networks and convergent pathways for ASD risk genes in neuronal environments.
Methodology Details: This experimental approach leverages proximity-dependent biotin identification (BioID2) to map the protein interactome of ASD risk genes in their native neuronal context [17].
Step 1: Experimental Design and Proteomic Profiling
- Select 41 ASD risk genes for characterization in primary neuronal cultures.
- Express each risk gene as a fusion construct with the BioID2 promiscuous biotin ligase.
- Allow expression for 24 hours in neuronal cultures to enable proximity-dependent biotinylation of interacting proteins.
- Harvest cells and affinity-purify biotinylated proteins using streptavidin beads.
- Identify interacting proteins via liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Step 2: Bioinformatics and Network Analysis
- Construct protein-protein interaction networks from mass spectrometry data.
- Analyze the resulting networks for functional convergence using overrepresentation and gene set enrichment analyses.
- Assess the impact of de novo missense variants from ASD patients on PPI networks.
- Perform CRISPR knockout of selected risk genes to validate functional associations.
- Correlate network properties with clinical behavior scores from ASD patients.
Key Findings: This neuron-specific mapping revealed several convergent pathways including mitochondrial/metabolic processes, Wnt signaling, and MAPK signaling. The approach demonstrated that ASD-associated de novo missense variants significantly disrupt the identified PPI networks, and clustering based on network properties revealed gene groups corresponding to clinical behavior score severity [17].
Table 2: Research Reagent Solutions for Neuron-Specific PPI Mapping
| Reagent/Resource | Function/Application | Key Specifications |
|---|---|---|
| BioID2 | Proximity-dependent biotin ligase for labeling interacting proteins | Engineered for higher efficiency and smaller size than original BioID |
| Primary Neuronal Cultures | Physiological context for interaction mapping | Preferentially cortical or hippocampal neurons at relevant developmental stages |
| Streptavidin Beads | Affinity purification of biotinylated proteins | High-capacity magnetic beads for efficient capture |
| LC-MS/MS System | Protein identification and quantification | High-resolution mass spectrometer with nanoflow chromatography |
| CRISPR/Cas9 System | Gene knockout for functional validation | Neuron-optimized delivery (e.g., lentiviral, AAV) |
| STRING Database | Protein-protein interaction reference | Minimum interaction score 0.9 for high-confidence interactions [7] |
Protocol 3: Cross-Species Functional Relationship Network Construction
Objective: To build a brain-specific functional relationship network (FRN) integrating cross-species data for improved ASD gene prioritization.
Methodology Details: This approach employs Bayesian integration of diverse functional genomic data across human, mouse, and rat to construct a comprehensive FRN [66].
Step 1: Data Collection and Processing
- Collect 213 non-cancer brain tissue gene expression datasets from GEO across three species (human, mouse, rat).
- Process expression data through log2 transformation, missing value imputation (k-Nearest Neighbor method), and gene-level summarization.
- Obtain protein-protein interaction data from BIND, BioGRID, IntAct, MINT, and MIPS (30,800 non-redundant interactions).
- Calculate quantitative physical interaction scores for all protein isoform pairs using the SPRING algorithm.
- Extract phenotype annotations from the Mouse Genome Informatics (MGI) database.
Step 2: Bayesian Network Integration
- Utilize a two-layer Bayesian network to compute posterior probabilities of functional relationships.
- Apply the formula: P(FR|E₁,E₂,E₃,...,Eₙ) = (1/C)P(FR)∏ᵢ₌₁ⁿP(Eᵢ|FR)
- Generate a genome-wide functional relationship network covering 21,122 genes.
- Train a random forest classifier on high-confidence ASD genes (n=143) to prioritize additional candidates.
Interpretation: The cross-species integration strategy provides more complete functional coverage than human-only approaches, particularly for brain-specific processes where human tissue samples may be limited. The resulting FRN enabled effective prioritization of ASD candidate genes from sequencing studies and identified key pathways involved in early neural development [66].
Cross-Species Functional Relationship Network Construction
Advanced Analytical Frameworks and Visualization
Weighted Gene Co-expression Network Analysis (WGCNA) for ASD
Objective: To identify modules of co-expressed genes in ASD-relevant neural cells and tissues.
Methodology Details: WGCNA identifies clusters of highly correlated genes across samples, providing a complementary approach to physical interaction networks for detecting functional modules [7].
Step 1: Network Construction
- Filter gene expression matrix to remove lowly expressed genes using the goodSampleGene function.
- Select a soft-thresholding power that satisfies the scale-free topology criterion (typically β=6-12 for signed networks).
- Calculate adjacency matrix using the chosen power value, then transform to topological overlap matrix (TOM).
- Perform hierarchical clustering on TOM-based dissimilarity matrix.
Step 2: Module Detection and Analysis
- Identify co-expression modules using dynamic tree cutting with minimum module size of 30 genes.
- Calculate module eigengenes (first principal component) representing overall expression patterns.
- Merge highly correlated modules (|correlation| > 0.9).
- Identify hub genes based on high module membership (MM > 0.9) and gene significance (GS > 0.2).
- Perform functional enrichment analysis on each module using clusterProfiler.
Application Example: In a study of Pitt-Hopkins syndrome (a monogenic ASD), WGCNA of neural progenitor cells and neurons revealed co-expression modules enriched for synaptic transmission, membrane excitability, and cell adhesion processes. Hub genes included histone modification components and synaptic vesicle trafficking proteins, suggesting novel mechanisms in ASD pathogenesis [7].
Visualization and Interpretation of Enrichment Results
Effective visualization is crucial for interpreting functional enrichment results, particularly given the complexity of ASD-associated pathways:
- Enrichment Map: Organizes enriched terms into a network with edges connecting overlapping gene sets, enabling identification of functional modules. Mutually overlapping gene sets cluster together, making it easier to identify major functional themes in the results [74] [72].
- Gene-Concept Network (cnetplot): Depicts linkages between genes and biological concepts as a network, particularly valuable for showing how individual ASD risk genes participate in multiple related pathways [74].
- Bar Plot and Dot Plot: Standard visualizations for displaying enrichment scores (p-values) and gene counts per term, with dot plots additionally encoding a second score (e.g., gene ratio) as dot size [74].
- Tree Plot: Performs hierarchical clustering of enriched terms based on similarity (Jaccard index or semantic similarity), then cuts the tree into subtrees labeled with high-frequency words to reduce complexity [74].
Emerging approaches include the use of large language models (LLMs) to assist in functional interpretation. Recent evaluations indicate that GPT-4 can generate common functions for gene sets with high specificity, providing complementary insights to traditional enrichment methods, though requiring careful validation to mitigate potential "hallucinations" [73].
Validation and Best Practices
Technical Validation Strategies
Robust validation of FEA results requires multiple complementary approaches:
- Statistical Robustness: Apply appropriate multiple testing corrections (Benjamini-Hochberg FDR, Bonferroni, or g:SCS) to account for the numerous hypotheses tested simultaneously during enrichment analysis [69].
- Experimental Validation: Where feasible, confirm key findings using orthogonal methods. For example, CRISPR knockout of hub genes identified through network analysis can validate their functional importance in ASD-relevant processes like mitochondrial function [17].
- Cross-dataset Validation: Test the reproducibility of identified pathways across independent ASD cohorts and datasets. A pathway identified consistently across multiple studies represents a more robust finding than one observed in a single dataset [30] [66].
Common Pitfalls and Mitigation Strategies
Table 3: Troubleshooting Functional Enrichment Analysis in ASD Research
| Challenge | Potential Impact | Mitigation Strategy |
|---|---|---|
| Inappropriate background set | Inflated or deflated enrichment significance | Use brain-expressed genes or cell type-specific backgrounds rather than whole genome |
| Incomplete annotation bias | Missing relevant ASD pathways | Combine multiple databases (GO, KEGG, Reactome, custom neural sets) |
| Network quality issues | Spurious module detection | Use high-confidence interaction data (e.g., STRING score >0.9) [7] |
| Multiple testing artifacts | False positive findings | Apply stringent FDR correction (≤0.05) and independent validation |
| Data type mismatch | Misleading interpretations | Match analysis type to data (ORA for gene lists, GSEA for expression rankings) [69] |
Functional Enrichment Analysis represents an indispensable methodology for validating the biological relevance of gene modules identified through ASD network topology research. By applying the protocols and best practices outlined in this technical guide—including network propagation approaches, neuron-specific interaction mapping, cross-species functional network integration, and advanced co-expression analysis—researchers can effectively translate complex genetic findings into coherent biological insights. The ongoing development of more sophisticated tools, including topology-aware enrichment methods and LLM-assisted interpretation, promises to further enhance our ability to decipher the complex molecular architecture of autism spectrum disorder. As these methodologies continue to evolve, they will increasingly enable the identification of convergent pathways and therapeutic targets from genetically heterogeneous ASD risk genes, ultimately accelerating the development of targeted interventions for this complex neurodevelopmental condition.
The exploration of the genetic architecture underlying cognitive traits represents a frontier in complex trait genomics. Research into the genetic correlations between intelligence and educational attainment (EA) has revealed a shared genetic etiology that provides a powerful framework for understanding human cognitive development and its relationship to a spectrum of neurodevelopmental conditions. Within the specific context of Autism Spectrum Disorder (ASD) risk genes research, investigating this shared architecture offers unprecedented opportunities to deconvolve the phenotypic heterogeneity of autism and identify coherent biological pathways. This whitepaper synthesizes current findings on the genetic correlations between cognitive traits and examines their interplay with the network topology of ASD risk genes, providing methodologies and analytical frameworks for researchers investigating the genetic foundations of neurodevelopment.
Genetic Architecture of Cognitive Traits
Definitions and Measurement Approaches
Intelligence is psychometrically defined as a general mental capacity encompassing reasoning, novel problem-solving, abstract thinking, and rapid learning abilities [75]. Modern assessment typically employs a hierarchical model with a general factor ("g") at the apex, underlying broader cognitive domains, which in turn comprise specific abilities. Educational attainment (EA), often operationalized as years of schooling, represents a related but distinct phenotype that captures both cognitive and non-cognitive factors influencing educational progression [75].
Table 1: Key Cognitive Traits and Their Measurement
| Trait | Definition | Primary Measurement | Heritability Estimates |
|---|---|---|---|
| Intelligence | General mental capacity for reasoning, problem-solving, abstract thinking | Psychometric test batteries (e.g., IS-T 2000R) | 20% (early childhood) to >60% (adulthood) [75] |
| Educational Attainment (EA) | Years of schooling completed | Self-report or administrative records | ~40% [75] |
| Childhood Cognitive Function | Cognitive abilities measured specifically in youth | Age-appropriate cognitive testing | SNP-based heritability: 27.3% [76] |
| Cognitive Performance | Domain-specific cognitive abilities | Task-based cognitive assessments | Varies by domain; highly polygenic [77] |
Heritability and Developmental Dynamics
Genetic influences on intelligence demonstrate dynamic changes across the lifespan. While shared environmental factors predominantly explain variance in early childhood, their influence diminishes during development, with genetic factors increasing in importance from approximately 20% in early childhood to over 60% in adulthood [75]. This increasing heritability is attributed to active genotype-environment correlation, wherein individuals selectively seek environments aligned with their genetic predispositions, creating feedback loops that amplify initial genetic advantages—a process termed genotype-environment transaction [75].
The heritability of intelligence further interacts with socioeconomic status, exhibiting higher estimates under more favorable circumstances (Scarr-Rowe effect) [75]. This gene-environment interaction highlights the context-dependent nature of genetic influences on cognitive development.
Molecular Genetic Foundations
Genome-Wide Association Studies and Polygenic Scores
Genome-wide association studies (GWAS) have transformed our understanding of the genetic architecture of cognitive traits. Early GWAS attempts with limited sample sizes yielded few replicable findings, but increasingly large meta-analyses have identified hundreds of significant loci.
Table 2: Evolution of GWAS for Cognitive Traits
| Study | Sample Size | Significant Loci | Explained Variance | Key Findings |
|---|---|---|---|---|
| EA1 (2013) | 100,000 | 3 SNPs | ~2% (via PGS) | First robustly replicable associations for EA [75] |
| EA2 (2016) | 300,000 | 74 independent SNPs | 3.2% (trait variance) | PGS predicted 4% of intelligence variance [75] |
| EA3 (2018) | 1,000,000 | 1,271 independent SNPs | 3.2-12.7% (EA); 5.1-9.7% (intelligence) | Substantial increase in predictive power [75] |
| EA4 (2023) | 3,000,000 | 3,952 SNPs | 7.0-15.8% (EA) | Top PGS decile: 60% university degree vs. 10% in bottom [75] |
| IQ2 (2017) | 78,000 | 18 gene loci | N/A | First robust genetic associations for intelligence [75] |
| IQ3 (2018) | 280,000 | 206 independent loci | 5.2% (trait variance) | Proof of principle for intelligence genetics [75] |
Polygenic scores (PGS) aggregate the effects of thousands of genetic variants across the genome to quantify an individual's genetic predisposition for a trait. For EA, PGS calculated from the largest GWAS explains approximately 13.3% of variance in educational outcomes among individuals of European ancestry [75]. The predictive power of PGS has increased substantially with sample size, demonstrating the highly polygenic nature of cognitive traits.
Genetic Correlations Between Cognitive Traits
Genetic correlation (rg) analyses reveal substantial shared genetic architecture between intelligence and EA, with estimates reaching approximately rg = 0.70 [75]. This high genetic correlation enables the use of EA—more easily measured in large cohorts—as a proxy for cognitive traits in genetic studies. Beyond their relationship with each other, cognitive traits show genetic correlations with diverse health outcomes:
- Longevity: Childhood cognitive function shows a genetic correlation of rg = 0.35 with parental longevity, suggesting shared genetic factors influencing both intelligence and lifespan [76].
- Health Behaviors: Higher PGS for EA and cognition correlate with healthier dietary patterns, particularly increased fruit consumption [77].
- Neuropsychiatric Conditions: Delay discounting (impulsive decision-making) shows genetic correlations with 73 traits including substance use, depression, and metabolic health issues [78].
Figure 1: Genetic Correlation Network of Cognitive Traits. This diagram illustrates the shared genetic architecture between intelligence, educational attainment, and various health and neurodevelopmental outcomes, including ASD. Genetic correlations (rg) represent the extent of shared genetic influences.
Methodological Approaches for Genetic Correlation Analysis
Core Analytical Frameworks
Linkage Disequilibrium Score Regression (LDSC): LDSC estimates genetic correlations using GWAS summary statistics by examining the relationship between test statistics and linkage disequilibrium (LD) scores [76]. This approach allows for the estimation of genetic covariances between traits without requiring individual-level genotype data. The method is particularly valuable for distinguishing polygenic signal from confounding biases, with intercepts near 1.0 indicating minimal confounding [76].
Polygenic Score Analysis: PGS calculation involves several key steps: (1) effect size estimation from a base GWAS, (2) clumping to retain independent SNPs, (3) calculation of weighted sums of alleles in the target sample, and (4) validation of predictive accuracy [77] [75]. For cognitive traits, PGS computed at different p-value thresholds (PT) can optimize prediction for specific outcomes.
Genomic Structural Equation Modeling (Genomic SEM): This extension of traditional SEM incorporates genetic covariance matrices from multiple GWAS to test complex models of genetic relationships between traits [79]. Genomic SEM can partition genetic variance into shared and trait-specific components, enabling researchers to determine whether genetic associations with educational fields persist after controlling for general educational attainment [79].
Experimental Protocols for Functional Validation
Transcriptomic Analysis of Neuronal Models: To bridge genetic associations with biological mechanisms in neurodevelopment, researchers can employ primary neuronal culture systems. One established protocol involves:
- Neuronal Culture Preparation: Isolate cortical neurons from E16.5 embryonic mouse tissue to generate highly pure neuronal populations [33].
- Gene Knockdown: At 5 days in vitro (DIV), infect neurons with lentivirus containing shRNA for partial depletion of ASD risk genes (e.g., ASH1L, CHD8, TBR1) [33].
- Transcriptomic Profiling: At DIV 10, perform RNA-sequencing to identify differentially expressed genes (DEGs) following target depletion [33].
- Functional Assessment: Use multielectrode array (MEA) recording to quantify changes in neuronal firing patterns throughout maturation [33].
Table 3: Research Reagent Solutions for Functional Genomics
| Reagent/Resource | Function | Application in Cognitive Genetics Research |
|---|---|---|
| Lentiviral shRNA | Targeted gene knockdown | Partial depletion of ASD risk genes in neuronal models to study functional consequences [33] |
| Primary Neuronal Cultures | Highly pure neuron populations | Controlled system for testing genetic effects without confounding cellular heterogeneity [33] |
| Multielectrode Arrays (MEA) | Recording neuronal firing patterns | Functional assessment of neuronal activity following genetic manipulation [33] |
| Connectivity Map (CMap) | Drug reversal prediction | Identifying potential therapeutic compounds that reverse ASD-related gene expression signatures [26] |
| STRING Database | Protein-protein interaction networks | Mapping molecular relationships between ASD risk genes and their protein products [26] |
Integrative Network Analysis Pipeline: A comprehensive approach to bridging transcriptomic discoveries with clinical applications involves:
- Differentially Expressed Gene Identification: Analyze microarray or RNA-seq data using linear modeling (e.g., limma R package) with thresholds of |log2FC| > 1.5 and FDR < 0.05 [26].
- Functional Enrichment Analysis: Conduct Gene Ontology (GO) and KEGG pathway analysis using clusterProfiler to identify biological processes disrupted in ASD [26].
- Protein-Protein Interaction Network Construction: Use STRING database (confidence score ≥ 0.4) and Cytoscape for visualization of molecular networks [26].
- Machine Learning Feature Selection: Apply random forest algorithms (ntree = 500) to identify top feature genes (e.g., SHANK3, NLRP3) with highest importance for ASD prediction [26].
- Immune Infiltration Analysis: Perform immune deconvolution using GSVA to correlate key genes with immune cell subpopulations [26].
- Diagnostic Validation: Evaluate diagnostic performance of top genes using ROC curve analysis, with AUC > 0.7 indicating good discriminatory power [26].
Figure 2: Genetic Correlation Analysis Workflow. This diagram outlines the computational and experimental pipeline for identifying and validating genetic correlations between cognitive traits and their functional consequences in neural systems.
ASD Risk Genes and Cognitive Trait Correlations
Phenotypic Decomposition and Genetic Programs
Recent advances in decomposing ASD heterogeneity have revealed distinct phenotypic classes with specific genetic correlates. A generative mixture modeling approach applied to 5,392 individuals with ASD identified four robust phenotypic classes [3]:
- Social/Behavioral (n = 1,976): High scores in social communication deficits, restricted/repetitive behaviors, with co-occurring disruptive behavior, attention deficit, and anxiety, but without developmental delays.
- Mixed ASD with DD (n = 1,002): Nuanced presentation with enriched developmental delays and specific repetitive behaviors.
- Moderate Challenges (n = 1,860): Consistently lower scores across all difficulty domains while maintaining ASD diagnosis.
- Broadly Affected (n = 554): Severe difficulties across all seven phenotypic categories.
These phenotypic classes demonstrate distinct patterns of co-occurring conditions, cognitive abilities, and developmental trajectories, with the broadly affected class showing significant enrichment for intellectual disability and early diagnosis [3]. Most importantly, these phenotypic divisions correspond to specific genetic programs, with class-specific differences in the developmental timing of affected genes aligning with clinical outcomes [3].
Convergent Molecular Pathways
Investigation of ASD risk genes encoding transcriptional regulators reveals convergent effects on neuronal gene expression despite diverse molecular functions. Studies of nine ASD-linked transcriptional regulators (ASH1L, CHD8, DNMT3A, KDM6B, KMT2C, MBD5, MED13L, SETD5, TBR1) found that despite targeting distinct chromatin modifications, these factors produce shared gene expression signatures in neurons, particularly disrupting critical synaptic genes [33]. This convergence extends to functional outcomes, with each transcriptional regulator affecting spiking and bursting patterns throughout neuronal maturation [33].
Network analyses further identify key hub genes in ASD pathophysiology, with random forest algorithms identifying ten feature genes (SHANK3, NLRP3, SERAC1, TUBB2A, MGAT4C, TFAP2A, EVC, GABRE, TRAK1, and GPR161) with highest importance for ASD prediction [26]. Among these, MGAT4C shows particularly strong discriminatory power (AUC = 0.730) as a potential biomarker [26].
Structural Brain Correlates
Genetic influences on cognitive traits in ASD manifest in early brain development. Neonates with higher autism polygenic scores show significant increases in fiber-bundle cross-section in the left superior corona radiata, a region crucial for motor and cognitive functions [80]. These structural differences emerge at birth, suggesting that genetic risk factors shape early white matter organization before behavioral symptoms emerge.
Gene-set enrichment analysis indicates that autism-associated variants linked to these white matter alterations are overrepresented in genes related to neuronal connectivity and synaptic function, including MAPT, KCNN2, and DSCAM [80]. This connection between genetic risk, brain structure, and cognitive function provides a mechanistic bridge between molecular findings and phenotypic outcomes.
Implications for Therapeutic Development
The decomposition of ASD heterogeneity into biologically meaningful subtypes enables targeted therapeutic development. Connectivity Map (CMap) analysis can predict potential therapeutics that reverse ASD-related gene expression signatures, with several candidates consistent with clinical trial results [26]. The identification of specific genetic programs underlying phenotypic classes allows for personalized intervention strategies based on an individual's genetic and phenotypic profile.
Furthermore, the shared genetic architecture between cognitive traits and ASD risk suggests that pathways regulating typical cognitive development may be disrupted in neurodevelopmental conditions. Therapeutic strategies that target these core pathways may address both core symptoms and associated cognitive features in ASD.
The integration of genetic correlation data with network topological analyses of ASD risk genes provides a powerful framework for understanding the complex relationship between typical cognitive variation and neurodevelopmental conditions, ultimately facilitating the development of more effective, personalized interventions.
Within the context of Autism Spectrum Disorder (ASD) risk genes network topology research, the identification of hub genes—highly connected genes in molecular networks—has emerged as a pivotal strategy for pinpointing robust diagnostic biomarkers [26]. The Receiver Operating Characteristic (ROC) curve analysis serves as the statistical cornerstone for evaluating the discriminatory power of these potential biomarkers, providing a rigorous framework to assess their ability to distinguish individuals with ASD from healthy controls [81] [82]. This technical guide details the integrated application of network analysis and ROC evaluation, creating a standardized pipeline for translating complex genetic findings into clinically actionable diagnostic tools.
The fundamental principle underlying this approach is that diseases like ASD, characterized by high clinical and genetic heterogeneity, often arise from perturbations in highly interconnected molecular networks [26]. Genes occupying central positions (hubs) within these networks theoretically exert greater influence on biological processes, making them prime candidates for diagnostic biomarkers. ROC curve analysis then provides the quantitative rigor to empirically test this hypothesis by evaluating each hub gene's true positive rate (sensitivity) against its false positive rate (1-specificity) across all potential expression thresholds [81] [82].
Methodological Framework
Hub Gene Identification Through Network Analysis
The initial phase involves constructing molecular networks from high-throughput genomic data to identify hub genes. The following workflow outlines this systematic process:
Figure 1: Integrated workflow for identifying and validating diagnostic hub genes through network analysis and ROC evaluation.
Data Acquisition and Preprocessing: Begin with acquiring transcriptomic data from repositories such as the Gene Expression Omnibus (GEO). For ASD research, dataset GSE18123 has been frequently utilized, containing peripheral blood or brain tissue samples from ASD patients and healthy controls [83] [26]. Preprocessing steps include background correction, normalization, and batch effect removal using R packages like limma [26].
Differential Expression Analysis: Identify Differentially Expressed Genes (DEGs) using the limma R package with standardized thresholds (e.g., |log2FC| > 0.5-1.5 and adjusted p-value < 0.05) [84] [85]. This step filters genes with significant expression changes between ASD and control groups.
Network Construction: Two primary methods are employed:
- Weighted Gene Co-expression Network Analysis (WGCNA): Constructs co-expression modules using the
WGCNAR package. The soft-thresholding power is typically set to 2-5, with a minimum module size of 30 genes [83] [84]. - Protein-Protein Interaction (PPI) Networks: Utilize databases like STRING (confidence score ≥ 0.4) and visualize networks using Cytoscape [26].
Hub Gene Identification: Apply multiple machine learning algorithms to refine hub gene selection:
- Random Forest: Implemented with the
randomForestR package (ntree=500) to rank genes by importance using MeanDecreaseGini [26]. - SVM-Recursive Feature Elimination (SVM-RFE): Executed via the
e1071package to iteratively remove features with minimal classification weights [84]. - LASSO Regression: Applied using the
glmnetpackage to perform feature selection with regularization [84].
ROC Curve Analysis: Principles and Implementation
ROC curve analysis quantitatively evaluates the diagnostic performance of identified hub genes by measuring their ability to classify ASD versus control samples across all possible expression thresholds [81].
Theoretical Foundation: The ROC curve plots the True Positive Rate (Sensitivity) against the False Positive Rate (1-Specificity) at various threshold settings [81] [82]. The key components include:
- Sensitivity: Probability of a positive test result for persons with the condition (True Positive Rate)
- Specificity: Probability of a negative test result for persons without the condition (True Negative Rate)
- Area Under the Curve (AUC): Measure of overall discriminative ability, ranging from 0.5 (no discrimination) to 1.0 (perfect discrimination) [81]
Calculation Methods: The AUC can be calculated parametrically under binormal distributions or nonparametrically using Wilcoxon statistics [82]. For non-Gaussian data with small sample sizes, the nonparametric approach is preferred [82].
Optimal Cut-Point Determination: Several methods exist for identifying the optimal threshold that maximizes both sensitivity and specificity:
- Youden Index: Maximizes (sensitivity + specificity - 1)
- Euclidean Index: Minimizes distance to the ideal point (0,1) on ROC plot
- Product Method: Maximizes the product of sensitivity and specificity [82]
Table 1: Interpretation Guidelines for AUC Values in Diagnostic Biomarker Research
| AUC Range | Diagnostic Discrimination | Clinical Utility |
|---|---|---|
| 0.90-1.00 | Excellent | High |
| 0.80-0.90 | Good | Potentially useful |
| 0.70-0.80 | Fair | Moderate, may require combination with other markers |
| 0.60-0.70 | Poor | Limited |
| 0.50-0.60 | Fail | None |
Implementation is facilitated by R packages such as pROC for calculating AUC and determining optimal cut-points [26] [84]. The following code provides a basic implementation framework:
Applications in ASD Research
Case Studies and Validation
Multiple studies have successfully implemented this integrated framework to identify and validate diagnostic biomarkers for ASD:
Immune-Related Hub Genes in ASD: A bioinformatics analysis identified FABP2 and JAK2 as hub genes through WGCNA. ROC analysis demonstrated their diagnostic potential with AUC values providing acceptable to excellent discrimination [83]. Immune infiltration analysis using CIBERSORT revealed significant correlations between these hub genes and immune cell subpopulations, suggesting their involvement in ASD immunopathology [83].
Machine Learning-Driven Biomarker Discovery: Another study integrated multiple machine learning algorithms (SVM-RFE, Random Forest, LASSO) to identify four hub genes (NEUROD6, NMU, PVALB, NECAB1) from prefrontal cortex samples [84]. The ROC analysis validated their diagnostic utility, with a nomogram model incorporating all four genes showing excellent discrimination (AUC > 0.9) in independent validation datasets [84].
HIF1A Pathway Hub Genes: Research focusing on the HIF1A pathway in ASD pathogenesis identified CDKN1A, ETS2, LYN, and SLC16A3 as diagnostic hub genes through machine learning approaches [85]. Single-cell RNA sequencing analysis pinpointed activated microglia as key immune cells expressing these genes. ROC curve analysis confirmed their strong diagnostic performance, with experimental validation in ASD mouse models providing biological credibility [85].
Multi-Gene Panel Development: A comprehensive analysis identified ten key feature genes (SHANK3, NLRP3, SERAC1, TUBB2A, MGAT4C, TFAP2A, EVC, GABRE, TRAK1, and GPR161) using random forest analysis [26]. ROC analysis indicated that most top genes had strong discriminatory power, particularly MGAT4C (AUC = 0.730), highlighting its potential as a robust biomarker [26].
Table 2: Experimentally Validated Hub Genes for ASD Diagnosis via ROC Analysis
| Hub Gene | AUC Value | Biological Function | Validation Method |
|---|---|---|---|
| FABP2 | Not specified | Fatty acid binding and metabolic processes | Gene expression validation in peripheral blood [83] |
| JAK2 | Not specified | Cytokine receptor signaling and immune response | Gene expression validation in peripheral blood [83] |
| NEUROD6 | >0.9 (combined model) | Neuronal differentiation and development | qRT-PCR in rat PFC tissues [84] |
| NMU | >0.9 (combined model) | Neuropeptide signaling and immune modulation | qRT-PCR in rat PFC tissues [84] |
| CDKN1A | Strong diagnostic performance | Cell cycle regulation and hypoxia response | Western Blot, qPCR in ASD mice and microglia [85] |
| MGAT4C | 0.730 | Glycosylation enzyme function | Random forest feature importance [26] |
Advanced Analytical Techniques
Immune Infiltration Analysis: The relationship between hub genes and immune cell populations can be investigated using deconvolution algorithms like CIBERSORT or ssGSEA [83] [84]. These methods estimate the proportion of different immune cells from bulk transcriptomic data, allowing correlation analysis between hub gene expression and immune cell infiltration [83].
Single-Cell Validation: Single-cell RNA sequencing analysis pinpoints specific cell clusters expressing the identified hub genes, with microglia frequently emerging as crucial immune cells in ASD pathophysiology [85].
Nomogram Construction: For clinical translation, nomogram models integrate multiple hub genes to predict individual disease risk. These models are evaluated using calibration curves and decision curve analysis to assess practical clinical utility [84].
The Scientist's Toolkit
Table 3: Essential Research Reagent Solutions for Hub Gene Discovery and Validation
| Reagent/Resource | Function | Example Applications |
|---|---|---|
| GEO Datasets (e.g., GSE18123) | Source of transcriptomic data for initial discovery | Differential expression analysis between ASD and control samples [83] [26] |
| STRING Database | Construction of protein-protein interaction networks | Identifying functional relationships between candidate genes [26] |
| CIBERSORT Algorithm | Deconvolution of immune cell fractions from bulk RNA data | Correlation analysis between hub genes and immune infiltration [83] |
| pROC R Package (v1.19.0.1+) | ROC curve analysis and AUC calculation | Determining diagnostic accuracy of hub genes [26] [84] |
| WGCNA R Package | Construction of weighted gene co-expression networks | Identifying co-expression modules and intramodular hub genes [83] [84] |
| Random Forest R Package (v4.7-1.2+) | Machine learning-based feature selection | Ranking genes by importance for ASD prediction [26] |
| Cytoscape Software (v3.10.3+) | Visualization and analysis of molecular interaction networks | PPI network construction and hub gene identification [26] |
| GeneCards Database | Compendium of gene annotations and functional information | Retrieving disease-related genes and functional annotations [85] |
Signaling Pathways and Experimental Framework
The molecular pathways underlying ASD pathogenesis provide biological context for hub gene function. The following diagram illustrates key signaling pathways implicated in ASD and their relationship to validated hub genes:
Figure 2: Signaling pathways connecting genetic and immune factors to ASD pathogenesis through hub gene regulation. Validated hub genes (yellow) are influenced by the JUN/HIF1A pathway activation.
The integration of network topology analysis with rigorous ROC evaluation establishes a powerful framework for advancing precision diagnostics in ASD research. This systematic approach moves beyond mere differential expression to identify functionally significant hub genes within molecular networks, with ROC analysis providing the quantitative rigor necessary to evaluate their clinical potential. As research progresses, the validation of these biomarkers across diverse populations and their integration with other data types will be essential for developing clinically viable diagnostic tools that reflect the complex genetic architecture of ASD.
The convergence of network biology and advanced computational methods is forging new pathways in therapeutic development for complex disorders. Within the specific context of Autism Spectrum Disorder (ASD), where genetic heterogeneity presents a significant challenge, research into ASD risk genes network topology provides a critical framework for understanding disease etiology and identifying intervention points. The protein-protein interaction (PPI) networks formed by ASD risk genes reveal convergent biological pathways—including synaptic transmission, mitochondrial metabolism, and Wnt signaling—that are frequently disrupted in the disorder [17]. By mapping these networks, we can transition from a gene-centric view to a pathway-based understanding of ASD, enabling the identification of repurposed drug candidates that target key nodes within these dysregulated networks. This guide details the integrated computational and experimental methodologies for systematically evaluating such candidates, from initial in silico prediction to definitive in vivo validation.
Computational Discovery:In SilicoPrediction of Drug Candidates
The first phase involves using sophisticated graph-based machine learning models to rank existing drugs for their potential efficacy against ASD-associated network pathologies.
Foundation Models for Zero-Shot Drug Repurposing
Foundation models like TxGNN represent the state of the art for systematic drug repurposing. This model is trained on a massive medical knowledge graph encompassing 17,080 diseases and uses a graph neural network (GNN) with a metric learning module to rank drugs for diseases, even those with no existing treatments (zero-shot prediction) [86].
Key Experimental Protocol: TxGNN Model Workflow
- Knowledge Graph Construction: The model is built upon a comprehensive KG integrating diverse data types, including drug-protein interactions, disease-gene associations, and protein-protein interactions [86].
- Graph Neural Network Pretraining: A GNN is trained in a self-supervised manner on this KG to generate meaningful latent representations (embeddings) for all entities (e.g., drugs, diseases, proteins) [86].
- Metric Learning for Zero-Shot Prediction: For a query disease with no known drugs, a disease signature vector is created based on its local network topology. The model identifies diseases with similar signatures and adaptively aggregates their knowledge to generate predictions for the query disease [86].
- Prediction and Explanation: The model outputs a likelihood score for each drug-disease pair. An integrated Explainer module, using techniques like GraphMask, extracts a sparse subgraph of multi-hop paths from the KG to provide interpretable rationales for the prediction [86].
The TxGNN model has demonstrated a 49.2% improvement in indication prediction accuracy and a 35.1% improvement in contraindication prediction under stringent zero-shot evaluation compared to previous methods [86].
Network Topology-Based Classification with GNNs
An alternative approach focuses specifically on the network of ASD risk genes. This method involves building a gene network where genes are nodes, interactions are edges, and chromosome band locations are node features. Graph models are then trained to classify the autism risk association of new genes [58].
Key Experimental Protocol: Graph-Based ASD Gene Classification
- Dataset Curation: Integrate the SFARI dataset (containing gene associations, risk scores, and chromosome band locations) with a human Protein Interaction Network (PIN) dataset [58].
- Graph Definition: Construct a graph ( G = (V, E, C) ), where ( V ) is the set of genes (nodes), ( E ) is the set of gene interactions (edges), and ( C ) is the set of risk labels [58].
- Model Training and Validation: Train various GNN architectures (e.g., Graph Sage, Graph Convolutional Networks, Graph Transformer) on three tasks:
- Binary Risk Classification: Differentiating genes with associated risk from those without.
- Multi-class Risk Classification: Categorizing genes based on confidence scores (high, moderate, low association).
- Syndromic Gene Classification: Identifying genes associated with an overarching medical condition [58].
In benchmark studies, the Graph Sage model achieved 85.80% accuracy on binary risk classification and 81.68% accuracy on multi-class risk classification, demonstrating the utility of network topology and chromosome features for identifying ASD-relevant biology [58].
Table 1: Key In Silico Models for ASD Drug Repurposing
| Model Name | Core Methodology | Primary Application | Reported Performance |
|---|---|---|---|
| TxGNN [86] | Graph Neural Network on medical Knowledge Graph | Zero-shot drug indication/contraindication prediction across 17,080 diseases | 49.2% improvement in indication prediction accuracy vs. benchmarks |
| Graph Sage [58] | Graph Neural Network on ASD gene-protein network | Classification of ASD risk association for genes | 85.80% accuracy on binary risk classification |
Diagram 1: TxGNN's zero-shot drug repurposing and explanation workflow.
Biological Validation: From Candidate to Mechanism
Following computational prediction, candidates must be validated in biological systems to confirm their mechanism of action within the context of ASD network biology.
Neuron-Specific Protein Network Mapping
A critical step is to move beyond generic PPI networks to maps generated in biologically relevant contexts, such as primary neurons. This accounts for cell-type-specific interactions that might be missed in other systems [17].
Key Experimental Protocol: BioID2 for Proximity-Labeling Proteomics
- Cell System: Transfect primary mouse neurons with plasmids expressing ASD risk genes fused to the BioID2 promiscuous biotin ligase.
- Biotinylation: Incubate with biotin to allow the fusion protein to biotinylate proximal interacting proteins.
- Affinity Purification and Mass Spectrometry: Lyse cells and capture biotinylated proteins using streptavidin beads. Identify captured proteins via liquid chromatography-tandem mass spectrometry (LC-MS/MS).
- Network Analysis and Validation: Construct PPI networks from identified proteins. Analyze for pathway convergence. Validate key interactions through orthogonal methods (e.g., co-immunoprecipitation) and assess the impact of patient-derived de novo missense variants on network integrity [17].
This approach has revealed that ASD risk genes, including non-syndromic ones, converge on pathways like mitochondrial metabolism, and that clustering risk genes based on their PPI networks can correlate with clinical behavior scores [17].
2In VitroFunctional Assays
After identifying a candidate drug and its potential mechanistic pathway, targeted in vitro assays are essential.
- CRISPR Knockout/Knockdown: Modulating the expression of an ASD risk gene can reveal phenotypic consequences and whether a candidate drug can rescue them. For example, CRISPR knockout of specific risk genes in neuronal models has been linked to impaired mitochondrial function, a pathway implicated in ASD [17].
- High-Content Phenotypic Screening: Utilize patient-derived iPSC neurons in multi-parameter assays. Measure relevant endpoints such as synaptic density, neurite outgrowth, or calcium signaling to quantify the rescue effects of a repurposed drug candidate.
- Metabolic and Mitochondrial Assays: Given the strong link between ASD risk genes and mitochondrial dysfunction [17], assays measuring oxygen consumption rate (OCR), extracellular acidification rate (ECAR), and ATP production are highly relevant for validating drug effects on this core pathway.
Table 2: Essential Research Reagent Solutions for ASD Drug Repurposing
| Reagent / Material | Function / Application in Research | Specific Example / Context |
|---|---|---|
| SFARI Gene Dataset [58] | Provides curated ASD risk gene associations and syndromic information; used as features and labels for GNN models. | Contains gene symbols, confidence scores (1-3), and syndromic associations. |
| Protein Interaction Network (PIN) [58] [17] | Serves as the foundational scaffold (edges) for constructing the gene/protein graph for network analysis. | Human PIN data from sources like BioGRID or STRING. |
| BioID2 Proximity Labeling System [17] | Identifies protein-protein interactions in live cells (e.g., neurons) under near-physiological conditions. | Used to map neuron-specific interaction networks for 41 ASD risk genes. |
| Primary Neurons [17] | Biologically relevant cell system for mapping ASD-related PPI networks and testing drug candidates. | Primary mouse cortical neurons used for BioID2 mapping. |
| Mass Spectrometry [17] | Identifies and quantifies proteins isolated via BioID2; essential for constructing PPI networks. | LC-MS/MS used to identify biotinylated proteins in neuronal BioID2 screens. |
Diagram 2: Neuron-specific PPI mapping with BioID2 to identify drug targets.
Preclinical and Clinical Translation
The final stage involves assessing the therapeutic efficacy of the validated candidate in whole-organism models and designing clinical trials.
1In VivoAnimal Studies
Animal models, particularly mouse models bearing mutations in high-confidence ASD risk genes, are indispensable for evaluating behavioral rescue.
- Model Selection: Select murine models with construct validity (e.g., knock-out/knock-in of genes identified in network screens, such as Shank3 or Syngap1).
- Drug Administration and Behavioral Testing: Treat mice with the repurposed drug candidate and subject them to a battery of behavioral tests. These should be relevant to ASD core symptoms and may include:
- Social Interaction Tests: Three-chamber sociability test.
- Communication Assays: Ultrasonic vocalization recording.
- Repetitive Behavior Analysis: Marble burying or self-grooming assays.
- Cognitive and Anxiety Tests: Morris water maze, elevated plus maze.
- Ex Vivo Analysis: Post-behavioral testing, analyze brain tissue to confirm target engagement and mechanistic hypotheses (e.g., changes in synaptic protein levels, restored mitochondrial function, or normalized neuronal signaling pathways).
Clinical Trial Considerations for Repurposed Drugs
While drug repurposing accelerates development, it faces unique challenges.
- Regulatory Hurdles: Navigating new indications with existing drugs requires clear regulatory pathways. Agencies like the FDA require robust evidence for efficacy in the new indication, even if the drug is already approved [87].
- Intellectual Property and Commercialization: Securing patent protection for a new use of an old drug can be difficult, which may deter investment. Novel formulations or dosing regimens are common strategies to overcome this [88].
- Clinical Trial Design: Trials must be carefully designed to detect potentially subtle improvements in ASD symptoms. Use of validated, sensitive endpoints and consideration of patient sub-populations defined by shared network pathology (e.g., "mitochondrial ASD" subgroup) are critical for success [17].
Diagram 3: The integrated drug repurposing pipeline, from genes to trials.
The journey from in silico prediction to in vivo efficacy for repurposed drugs in ASD is a structured, multi-stage process firmly grounded in the network topology of risk genes. By leveraging GNNs on rich biological knowledge graphs, researchers can generate high-probability candidate drugs. Subsequent validation through neuron-specific proteomics and functional assays in physiologically relevant models confirms their mechanism of action and therapeutic potential. This integrated approach, which moves from computational screens to biological mechanism and finally to clinical relevance, offers a powerful strategy for rapidly delivering new treatment options by targeting the core network pathologies of ASD.
Conclusion
The application of network topology analysis has fundamentally advanced our understanding of ASD, moving the field beyond a collection of disparate risk genes toward a systems-level model of interconnected biological pathways. Key takeaways include the central role of networks in synaptic function, chromatin remodeling, and neuronal signaling; the power of computational methods like network propagation and WGCNA to identify core pathogenic modules; and the critical importance of accounting for genetic and phenotypic heterogeneity. The validation of these network-based insights through genetic correlations, functional analyses, and biomarker identification paves a concrete path for translation. Future research must focus on increasing the ancestral diversity of genetic datasets, integrating multi-omic data across single-cell resolutions, and moving promising in silico drug candidates into pre-clinical and clinical trials. This systems-level framework holds immense promise for delivering on the goals of precision medicine in ASD, ultimately leading to targeted therapies and improved diagnostic tools.
References
- 1. Rare variants and the oligogenic architecture of autism [https://www.sciencedirect.com/science/article/abs/pii/S0168952522000701]
- 2. How rare and common risk variation jointly affect liability for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8495987/]
- 3. Decomposition of phenotypic heterogeneity in autism ... [https://www.nature.com/articles/s41588-025-02224-z]
- 4. Polygenic and developmental profiles of autism differ by ... [https://www.nature.com/articles/s41586-025-09542-6]
- 5. Evaluation of familial phenotype deviation to measure the ... [https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-025-01532-7]
- 6. Genomic and Developmental Models to Predict Cognitive ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12012735/]
- 7. Gene network analysis identifies dysregulated pathways in ... [https://www.nature.com/articles/s41598-025-89334-0]
- 8. Integrated multi-omics analyses of synaptosomes revealed ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12532717/]
- 9. Signalling pathways in autism spectrum disorder [https://www.nature.com/articles/s41392-022-01081-0]
- 10. Synaptic, transcriptional, and chromatin genes disrupted in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4402723/]
- 11. Chromatin accessibility: biological functions, molecular ... [https://www.nature.com/articles/s41392-024-02030-9]
- 12. Exploring Chromatin Remodeling: New Progress in Gene ... [https://www.cd-genomics.com/blog/chromatin-remodeling-epigenetics-gene-regulation-detection-technology/]
- 13. Chromatin Modifiers in Transcriptional Regulation [https://pmc.ncbi.nlm.nih.gov/articles/PMC8084290/]
- 14. Network Structure Analysis Identifying Key Genes of Autism ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7125446/]
- 15. Neuronal protein interaction networks in autism spectrum ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10025519/]
- 16. Network Diffusion-Based Prioritization of Autism Risk Genes ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5622537/]
- 17. Neuron-specific protein network mapping of autism risk ... [https://www.sciencedirect.com/science/article/pii/S2211124722015522]
- 18. Protein interaction studies in human induced neurons ... [https://www.sciencedirect.com/science/article/pii/S2666979X22002130]
- 19. A Machine Learning Approach to Predicting Autism Risk ... [https://www.frontiersin.org/journals/genetics/articles/10.3389/fgene.2020.500064/full]
- 20. Dysregulation of G protein subunits in autism - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC12378886/]
- 21. Dysregulation of G protein subunits in autism: decreased ... [https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2025.1587727/full]
- 22. Major autism study uncovers biologically distinct subtypes, ... [https://www.princeton.edu/news/2025/07/09/major-autism-study-uncovers-biologically-distinct-subtypes-paving-way-precision]
- 23. Genomic Architecture of Autism From Comprehensive ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10726699/]
- 24. Genomic Data [https://www.sfari.org/resource/spark/spark-genomic-data/]
- 25. Autism Sequencing Consortium (ASC) [https://ega-archive.org/studies/phs000298]
- 26. Integrating Network Analysis and Machine Learning ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12469866/]
- 27. Advancing precision diagnosis in autism: Insights from ... [https://www.sciencedirect.com/science/article/pii/S101684782500072X]
- 28. Common genetic risk variants identified in the SPARK ... [https://www.nature.com/articles/s41398-020-00953-9]
- 29. How to visually interpret biological data using networks [https://pmc.ncbi.nlm.nih.gov/articles/PMC4154490/]
- 30. A network-based method for associating genes with autism ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10960359/]
- 31. Integrating omics data and protein interaction networks to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5601632/]
- 32. Integrating multi-omic data to identify key genes and ... [https://www.nature.com/articles/s41598-025-07334-6]
- 33. Autism spectrum disorder risk genes have convergent ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11974841/]
- 34. A convergent molecular network underlying autism and ... [https://www.sciencedirect.com/science/article/pii/S2405471221002878]
- 35. Multi-omics study reveals differential expression and ... [https://www.nature.com/articles/s41598-025-95860-8]
- 36. Transcriptomic Analysis of Autistic Brain Reveals ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3607626/]
- 37. Transcriptome analysis reveals dysregulation of innate ... [https://www.nature.com/articles/ncomms6748]
- 38. Integrative Functional Genomic Analyses Implicate Specific ... [https://www.sciencedirect.com/science/article/pii/S0092867413013494]
- 39. Enriched expression of genes associated with autism ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5802446/]
- 40. Autism spectrum disorder: pathogenesis, biomarker, and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10908366/]
- 41. Transcriptomic divergence of network hubs in the prenatal ... [https://www.nature.com/articles/s42003-025-08962-4]
- 42. Exploring the molecular mechanisms of comorbidity between ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12643707/]
- 43. A joint complex network and machine learning approach ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12588478/]
- 44. Autism Spectrum Disorder Markets Expected to Exhibit a ... [https://www.biospace.com/press-releases/autism-spectrum-disorder-markets-expected-to-exhibit-a-cagr-of-5-84-during-2025-2035-impelled-by-innovative-treatments]
- 45. Connectivity Map (CMAP) [https://www.broadinstitute.org/connectivity-map-cmap]
- 46. Decoding Connectivity Map-based drug repurposing for ... [https://pubmed.ncbi.nlm.nih.gov/37068308/]
- 47. Evaluation of connectivity map shows limited ... [https://www.nature.com/articles/s41598-021-97005-z]
- 48. In silico methods for drug-target interaction prediction - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12570315/]
- 49. GraphIX: Graph-based In silico XAI(explainable artificial ... [https://arxiv.org/abs/2212.10788]
- 50. Four New Autism Subtypes Link Genes to Children's Traits [https://www.scientificamerican.com/article/four-new-autism-subtypes-link-genes-to-childrens-traits/]
- 51. Decomposition of phenotypic heterogeneity in autism ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11343255/]
- 52. Random Forest Algorithm in Machine Learning [https://www.geeksforgeeks.org/machine-learning/random-forest-algorithm-in-machine-learning/]
- 53. Salience Network–Based Classification and Prediction of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3951904/]
- 54. MADE-for-ASD: A multi-atlas deep ensemble network ... [https://www.sciencedirect.com/science/article/pii/S0010482524011685]
- 55. Deep neural networks and deep deterministic policy ... [https://www.nature.com/articles/s41598-025-26854-9]
- 56. Make Your Network Maps Shine with 16 New Analyzer ... [https://visiblenetworklabs.com/2025/03/06/make-your-network-maps-shine-with-16-new-analyzer-color-palettes/]
- 57. Multi-omics reveals cross-tissue regulatory mechanisms of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12572420/]
- 58. Graph Node Classification to Predict Autism Risk in Genes [https://pmc.ncbi.nlm.nih.gov/articles/PMC11049455/]
- 59. Mitigating sex-related biases to elucidate the autism ... [https://pubmed.ncbi.nlm.nih.gov/40653186/]
- 60. Mitigating Sex-Related Biases to Elucidate the Autism ... [https://www.sciencedirect.com/science/article/abs/pii/S0006322325013125]
- 61. Discovery of obesity genes through cross-ancestry analysis [https://www.nature.com/articles/s41467-025-64933-7]
- 62. Autism heterogeneity related to preterm birth: multi- ... [https://www.sciencedirect.com/science/article/pii/S2667174325001685]
- 63. Forecasting risk gene discovery in autism with machine ... [https://www.nature.com/articles/s41598-020-61288-5]
- 64. A network-based method for associating genes with autism ... [https://www.frontiersin.org/journals/bioinformatics/articles/10.3389/fbinf.2024.1295600/full]
- 65. Predicting the Risk Genes of Autism Spectrum Disorders - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8236850/]
- 66. Brain-specific functional relationship networks inform ... [https://www.nature.com/articles/s41398-018-0098-6]
- 67. A hybrid Stacking-SMOTE model for optimizing the prediction ... [https://bmcbioinformatics.biomedcentral.com/articles/10.1186/s12859-023-05501-y]
- 68. De novo network analysis reveals autism causal genes ... [https://www.life-science-alliance.org/content/6/10/e202302142]
- 69. Nine quick tips for pathway enrichment analysis - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9371296/]
- 70. Integrated systems analysis reveals a molecular network ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4300495/]
- 71. GSEA [https://www.gsea-msigdb.org/]
- 72. Gene-set enrichment analysis and visualization on the web ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12373637/]
- 73. Enhancing functional gene set analysis with large ... [https://www.nature.com/articles/s41592-024-02526-w]
- 74. Chapter 15 Visualization of functional enrichment result [https://yulab-smu.top/biomedical-knowledge-mining-book/enrichplot.html]
- 75. The Genetics of Intelligence (24.01.2025) [https://di.aerzteblatt.de/int/archive/article/242633]
- 76. Genetic link discovered between childhood intelligence ... [https://www.eurekalert.org/news-releases/1102475]
- 77. Influences of genetic factors for educational attainment and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12493237/]
- 78. Genetic Study Links Impulsive Decision Making to a Wide ... [https://today.ucsd.edu/story/genetic-study-links-impulsive-decision-making-to-a-wide-range-of-health-and-psychiatric-risks]
- 79. Genetic associations with educational fields [https://www.nature.com/articles/s41588-025-02391-z]
- 80. Autism risk genes linked to white matter changes at birth, ... [https://www.news-medical.net/news/20250205/Autism-risk-genes-linked-to-white-matter-changes-at-birth-shaping-early-brain-development.aspx]
- 81. ROC curve analysis: a useful statistic multi-tool in the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11266376/]
- 82. Methods of determining optimal cut-point of diagnostic ... [https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-024-02198-2]
- 83. Bioinformatics analysis of genomic and immune infiltration ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9577768/]
- 84. Identification of hub genes and construction of diagnostic ... [https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2022.1032917/full]
- 85. Unraveling pathogenesis and potential biomarkers for ... [https://www.sciencedirect.com/science/article/pii/S0969996124003656]
- 86. A foundation model for clinician-centered drug repurposing [https://www.nature.com/articles/s41591-024-03233-x]
- 87. Therapeutic innovation in drug repurposing: Challenges ... [https://www.sciencedirect.com/science/article/pii/S1359644625001035]
- 88. From Lab to Clinic: Success Stories of Repurposed Drugs in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12535746/]